Abstract
Purpose
To evaluate the efficacy and toxicity of heptaplatin, paclitaxel, and 5-fluorouracil combination chemotherapy in patients with advanced gastric cancer.
Materials and Methods
Between July 2002 and September 2003, nineteen patients were enrolled in this study. Paclitaxel 135 mg/m2 iv on day 1, heptaplatin 400 mg/m2 iv on day 2 and 5-fluorouracil 800 mg/m2 on day 2~4 were administered and the regimen was repeated every 3 weeks.
Results
The median age of the patients was 60 years (range: 32~74) and the most common sites of metastasis were liver and lymph nodes. In the 16 evaluated patients, the overall response rate was 43.8%, but this was without any complete response. The median time to disease progression was 3.93 months (range: 0.26~8.1) and the median response duration for the 7 responding patients was 3.83 months (range: 1.48~6.07). The median overall survival for 19 patients was 7.01 months (range: 0.26~17.44). A median of 3 cycles (range: 1~7) and a total of 65 cycles were administered and evaluated for toxicity. The most common hematologic toxicities were NCI grade I/II anemia (47.7%), neutropenia (9.2%) and thrombocytopenia (6.2%). The most common non-hematologic toxicities more than grade II were nausea/vomiting (30.8%/9.2%). One elderly patient with ECOG 2 had a life-threatening complication of pneumonia.
Conclusion
The combination of heptaplatin, paclitaxel, and 5-fluorouracil showed significant activity and favorable toxicity profiles in patients with advanced gastric cancer. However, one elderly patient who had poor performance experienced a life-threatening toxicity/complication. Our results suggest that the efficacy of this combination chemotherapy can be maximized when administered to the patients with good performance status. Further studies with large numbers of patients and long-term follow-up study will be needed.
Despite its reduced incidence and mortality, gastric cancer remains one of the most common malignancies in Asia including Korea (1). Metastatic gastric cancer is regarded as an incurable disease. Several randomized studies with this disease setting have compared the best supportive care strategies using chemotherapy, and the studies have demonstrated that systemic treatment can, to a certain extent, improve overall survival and quality of life (2~5). Although several randomized phase III studies have failed to define a gold standard combination regimen for the palliation of gastric cancer (6~10), a combination of cisplatin and 5-fluorouracil (5-FU) is commonly used as the standard treatment and this has 30~50% activity in advanced gastric cancer (9,10). However, toxicities such as nausea, vomiting and nephrotoxicity still remain the major limitations for the patients receiving chemotherapy containing cisplatin. Heptaplatin (SKI-2053R, Sunpla®), the new derivative of platinum, was developed to reduce the nephrotoxicity of cisplatin (11,12). Dose-limiting side effects are mainly hepatotoxicity and myelosuppression. As a single therapeutic agent, the response rate was 17%, and the median response duration was 7.2 months in advanced gastric cancer (13). A combination of heptaplatin and 5-FU achieved a 30% response rate with a moderate toxicity profile in patients with gastric cancer (14).
Paclitaxel, the prototype taxane compound that interferes with tubulin assembly and disassembly, has been studied extensively in patients with gastric cancer. This agent has been combined with a variety of well-established compounds for the treatment of gastric cancer in a number of trials, and this combination has shown a considerable response rate (15~17). We performed a phase II trial to evaluate the efficacy and toxicity of heptaplatin, paclitaxel, and 5-fluorouracil combination chemotherapy in patients with advanced gastric cancer, and we report herein on the results of our study.
Histologically proven advanced gastric cancer patients were included in our study. Other patient eligibility criteria were the presence of measurable tumor lesions, an age >18 years, ECOG performance 0~2, adequate organ function, and a life expectancy of at least 3 months. The study patients had not received chemotherapy previously for their advanced disease and they had completed adjuvant chemotherapy more than 6 months before entry to our study. Radiotherapy was permitted for palliation, but not if it was associated with a present measurable lesion. All patients gave their written informed consent before enrollment in our study.
Paclitaxel 135 mg/m2 was administered in 500 ml of dextrose 5% as a 3-hour infusion on day 1, and heptaplatin 400 mg/m2 was administered in 500 ml of normal saline as a 3-hour infusion on day 2. 5-fluorouracil 800 mg/m2/day was administered in 1,000 ml of dextrose 5% as a 24 hour continuous infusion on day 2~4 and this regimen was repeated every 3 weeks. Treatment was continued until disease progression, unacceptable adverse effects, or withdrawal of consent by the patient was noted.
Treatment was delayed for a maximum of 14 days until the toxicity side effects were resolved. If there were second episodes of grade 2 toxicity or any grade 3 toxicity, a 20% reduction of the drug regimen was done. If there were third occurrences of grade 2 toxicity, second episodes of grade 3 toxicity or any grade 4 toxicity, a 40% reduction of the drug regimen was required (18). Fourth episodes of grade 2 toxicity, third episodes of grade 3 toxicity or second episodes of grade 4 toxicity, despite the dose reduction, required us to discontinue the treatment. In case of myelosuppression, the treatment was postponed or adjusted according to the following instructions: in the case that the WBC was <4,000/mm3 or platelets were <100,000/mm3 at the start of a cycle, treatment should be postponed for 1 week.
Pretreatment evaluation included a complete medical history and physical examination, a complete blood count, chemistry profile, chest X-ray and a radiologic tumor parameter assessment. Patients were assessed for their clinical response after 2 cycles of chemotherapy. Tumor response classification was derived from the standard World Health Organization criteria. Time to disease progression was defined as the interval from the date of enrollment to the first observation of progressive disease or the occurrence of death from any cause. The response duration was defined as the interval from the date of the first documented treatment response to the date of disease progression. Toxicities were assessed according to the National Cancer Institute of Canada Clinical Trials Group expanded common toxicity grading.
Nineteen patients were enrolled in this study between July 2002 and September 2003. Table 1 lists the demographic data and baseline characteristics for all patients. The median age of the patients was 60 years (range: 32~74) and most of the patients (84.2%) had ECOG 0-1. The most common sites of metastasis were liver and lymph nodes. Sixteen patients had one metastatic site and 3 patients had 2 or more sites of organ involvement. Eight patients (42.1%) had undergone subtotal or total gastrectomy. Two patients had received adjuvant chemotherapy with 5-FU based chemotherapy. Three patients were excluded from the response evaluation; two patients refused chemotherapy after one cycle of treatment and other patient had a life-threatening toxicity of pneumonia.
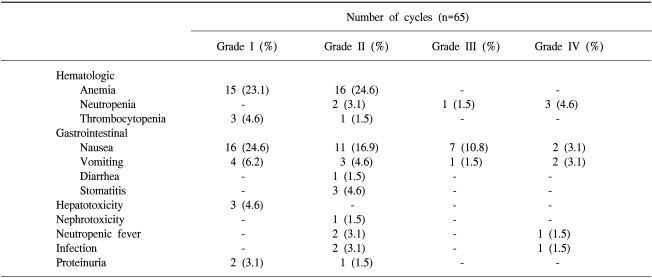
A median of 3 cycles per patient (range: 1~7) and a total of 65 cycles were administered and evaluated for toxicity. The incidence of toxicity was summarized in Table 2. The most common hematologic toxicities were NCI grade I/II anemia (47.7%), neutropenia (9.2%) and thrombocytopenia (6.2%). Although we did not measure the nadir of the CBC, only 4 patients developed ANC <500/mm3 and they recovered with G-CSF administration. There was no grade III/IV thrombocytopenia. The most common non-hematologic toxicities greater than grade II were nausea/vomiting (30.8%/9.2%). Three patients experienced mild proteinuria (+1~+2), but this resolved spontaneously. Patients suffering with massive proteinuria were not noted. Only one patient experienced an elevated creatinine level after chemotherapy, but this complication was considered due to the underlying bilateral hydronephrosis. The creatinine level was partially resolved after a double-J catheter was inserted, and heptaplatin administration was not continued for this patient. One elderly patient who had chronic obstructive lung disease with ECOG 2 developed severe neutropenia followed by life threatening pneumonia and sepsis.
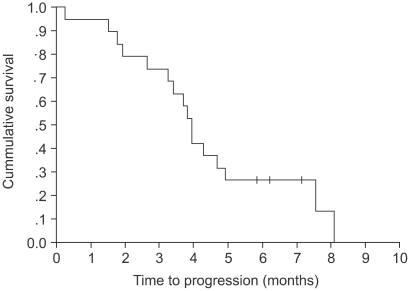
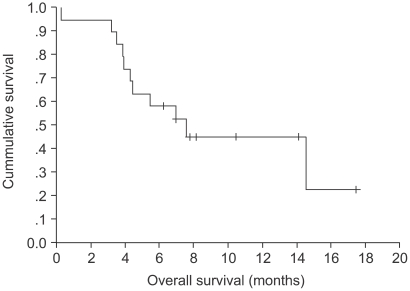
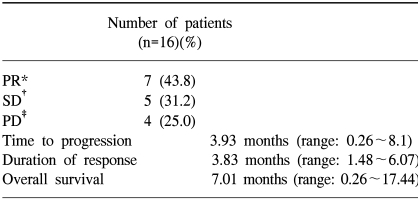
The response evaluation was assessed by an independent radiology review and the results are summarized in Table 3. Among the 16 evaluated patients, 7 patients achieved a partial response and no complete response was noted. The overall response rate was 43.8%. The median time to disease progression was 3.93 months (range: 0.26~8.1) (Fig. 1) and the median response duration for the 7 responding patients was 3.83 months (range: 1.48~6.07). The median follow-up duration for the surviving patients was 7.96 months (range: 6.2~17.4). The median overall survival for 19 patients was 7.01 months (range: 0.26~17.44), and the 1-year survival rate was 44.7% (Fig. 2). A total of 11 deaths were noted and 10 patients died from disease progression and one died from pneumonia and sepsis after chemotherapy.
We found that the combination chemotherapy of heptaplatin, paclitaxel, and infusional 5-FU was highly effective and well tolerated. Although there were no cases of complete response, a response rate of 43.75% was noted in this study. Given that the previous studies with cisplatin, paclitaxel and infused 5-FU reported a response rate of 46~65% (15~17), when we substituted heptaplatin for cisplatin in this trial, the response rate was comparable to a previous study using cisplatin, paclitaxel, and 5-FU combination chemotherapy. This finding suggests that the efficacy of heptaplatin is as good as that of cisplatin.
The toxicity profiles of heptaplatin, paclitaxel, and 5-FU were not serious. The most common hematologic toxicities were NCI grade I/II anemia, neutropenia and thrombocytopenia. Three of 4 neutropenic patients developed neutropenic fever, but this was well controlled by empirical antibiotics and G-CSF administration except for one patient. The most common non-hematologic toxicities greater than grade II were nausea/vomiting. However, only 3 out of 65 (4.6%) cycles developed grade III/IV vomiting. Considering that the previous studies with cisplatin-containing regimens produce greater than grade III nausea/vomiting in large numbers of patients, heptaplatin is well tolerated in terms of nausea/vomiting. A recent trial of heptaplatin, UFT-E and leucovorin in advanced gastric cancer patients also reported a low incidence of nausea/vomiting (19). In terms of nephrotoxicity, there were three cases of proteinuria after heptaplatin administration. Since the severity of the proteinuria was mild and resolved spontaneously, heptaplatin could be safely re-administered thereafter. One patient experienced an elevated creatinine level after the third cycle of chemotherapy, but the examination revealed that the renal dysfunction could be attributed to bilateral hydronephrosis caused by the periureteral metastasis of gastric cancer. This patient regained her renal function after insertion of a double J catheter. Further administration of heptaplatin was omitted from this patient's chemotherapy thereafter.
Although the response rate with haptaplatin, paclitaxel, and 5-FU combination therapy was high, the median time to progression and median overall survival was 3.93 months and 7.01 months, respectively which is lower than a previous study. This result may be attributed to the small number of patients. The objective results achievable with many of the established combinations are similar to our results regarding the response rate, but our results were very unsatisfactory in terms of patient survival. More active agents are clearly needed to improve the treatment options for gastric cancer in the palliative setting.
In our study, one elderly patient who had underlying chronic obstructive lung disease with ECOG 2 developed severe neutropenia after the first cycle of chemotherapy, and this was followed by pneumonia and sepsis. Another two patients with poor performance status refused further treatment after one cycle of chemotherapy due to toxicity. These findings indicate that this combination of chemotherapy should be cautiously administered to elderly patients or patients with a poor performance status. For this patient group, the new oral agents such as capecitabine or S-1 would play a role in the treatment of advanced gastric cancer (20,21).
The combination of heptaplatin, paclitaxel, and 5-fluorouracil showed significant activity and favorable toxicity for patients with advanced gastric cancer. However, one elderly patient who had a poor performance status experienced a life-threatening toxicity. Our results suggest that the efficacy of this combination chemotherapy can be maximized when administered to the patients with good performance status.
References
1. Bae JM, Won YJ, Jung KW, Park JG. Annual report of the Central Cancer Registry in Korea-2000: based on registered data from 131 hospitals. Cancer Res Treat. 2002; 34(2):77–83.
2. Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M. Randomized comparison of fluorouracil, epidoxorubicin and methotrexate plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer. 1995; 71:587–591. PMID: 7533517.
3. Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin and methotrexate in advanced gastric cancer. Cancer. 1993; 72:37–41. PMID: 8508427.

4. Glimelius B, Hoffman K, Haglund U, Nyren O, Sjoden PO. Initial of delayed chemotherapy with best supportive care in advanced gastric cancer. Ann Oncol. 1994; 5:189–190. PMID: 8186165.
5. In : Scheithauser W, Kome KG, Zeh B, editors. Palliative chemotherapy versus supportive care in patients with metastatic gastric cancer: a randomized trial. 1995. Second International Conference on Biology, Prevention and Treatment of GI Malignancy, Koln; Germany. 68 (Abstr).
6. Kelsen D, Atiq OT, Saltz L, Niedzwiecki D, Ginn D, Chapman D, Heelan R, Lightdale C, Vinciguerra V, Brennan M. FAMTX versus etoposide, doxorubicin and cisplatin: a random assignment trial in gastric cancer. J Clin Oncol. 1992; 10:541–548. PMID: 1548519.

7. Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, Planker M, Santos JG, Piedbois P, Paillot B, Bodenstein H, Schmoll HJ, Bleiberg H, Nordlinger B, Couvreur ML, Baron B, Wils JA. Final results of a randomized Phase III trial of sequential high-dose methotrexate, fluorouracil and doxorubicin versus etoposide, leucovorin and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer cooperative group. J Clin Oncol. 2000; 18:2648–2657. PMID: 10894863.
8. Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, Hughes M, Mansi J, Findlay M, Hill A, Oates J, Nicolson M, Hickish T, O'Brien M, Iveson T, Watson M, Underhill C, Wardley A, Meehan M. Randomized trial comparing epirubicin, cisplatin and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997; 15:261–267. PMID: 8996151.

9. Kim NK, Park YS, Heo DS, Suh C, Kim SY, Park KC, Kang YK, Shin DB, Kim HT, Kim HJ. A phase III randomized study of 5-fluorouracil and cisplatin versus 5-fluorouracil alone in the treatment of advanced gastric cancer. Cancer. 1993; 71:3813–3818. PMID: 8508349.
10. Shimada Y, Shirao K, Ohtsu A. A phase III study of UFT+MMC versus 55-FU+CDPP versus 5-FU alone in patients with advanced gastric cancer; JCOG study 9205. Proc ASCO. 1999; 18:272a. (Abstr).
11. Kim HT, Kim DK, Cho YB, Kim TS, Jung IH, Kim KH, Heo DS, Bang YJ, Shin SW, Kim NK. Influence of exposure and infusion times on the cytotoxicity and pharmacokinetics of cis-malonate [(4R,5R)-4,5-bis(aminomethyl)-2-isopropyl-1,3-dioxolane] platinum(II). Cancer Chemother Pharmacol. 1998; 41:109–116. PMID: 9443623.
12. Kim DK, Kim HT, Tai JH, Cho YB, Kim TS, Kim KH, Park JG, Hong WS. Pharmacokinetics and antitumor activity of a new platinum compound, cis-malonate [(4R,5R)-4,5-bis(aminomethyl)-2-isopropyl-1,3-dioxolane] platinum(II), as determined by ex vivo pharmacodynamics. Cancer Chemother Pharmacol. 1995; 35:1–6. PMID: 7497577.
13. Kim NK, Im SA, Kim DW, Lee MH, Jung CW, Cho EK, Lee JT, Ahn JS, Heo DS, Bang YJ. Phase II clinical trial of SKI-2053R, a new platinum analog, in the treatment of patients with advanced gastric adenocarcinoma. Cancer. 1999; 86:1109–1115. PMID: 10506693.

14. Kim NK, Bang Y, Heo DS, Kang Y, Kang WK, Kim H, Kim H, Kim JS, Kim HT, Roh JK. SKI 2053R and 5-fluorouracil (5-FU) in advanced gastric adenocarcinoma; phase II clinical trial. Proc AM Soc Clin Oncol. 2000; 19:323a. (Abstr. 1277).
15. Murad AM, Petroianu A, Guimaraes RC, Aragao BC, Cabral LO, Scalabrini-Neto AO. Phase II trial of the combination of paclitaxel and 5-fluorouracil in the treatment of advanced gastric cancer: a novel, safe, and effective regimen. Am J Clin Oncol. 1999; 22:580–586. PMID: 10597742.
16. Kim YH, Shin SW, Kim BS, Kim JH, Kim JG, Mok YJ, Kim CS, Rhyu HS, Hyun JH, Kim JS. Paclitaxel, 5-fluorouracil, and cisplatin combination chemotherapy in the treatment of advanced gastric carcinoma. Cancer. 1999; 85:295–301. PMID: 10023695.
17. Kollmannsberger C, Quietzsch D, Haag C, Lingenfelser T, Schroeder M, Hartmann JT, Baronius W, Hempel V, Clemens M, Kanz L, Bokemeyer C. A phase II study of paclitaxel, weekly, 24-hour continuous infusion 5-fluorouracil, FA and cisplatin in patients with advanced gastric cancer. Br J Cancer. 2000; 83:458–462. PMID: 10945491.
18. Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004; 22:23–30. PMID: 14665611.

19. Oh SC, Yoon SY, Seo JH, Choi CW, Kim BS, Shin SW, Kim YH, Kim SY, Yoon HJ, Cho KS, Kim JS. Pilot study of heptoplatin, UFT-E, and leucovorin in advanced gastric carcinoma. Cancer Res Treat. 2003; 35(2):117–122.
20. Koizumi W, Taguchi T. A phase II study of capecitabine (Xeloda) in patients with advanced-metastatic gastric carcinoma. Proc Am Soc Clin Oncol. 2001; 20:142b. (Abstr. 2320).
21. Koizumi W, Kurihara M, Nakano S, Hasegawa K. Phase II study of S-1, a novel oral derivative of 5-fluorouracil in advanced gastric cancer. Oncology. 2000; 58:191–197. PMID: 10765119.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download