Abstract
Purpose
To determine the efficacy and tolerability of a modified chronomodulated infusion of oxaliplatin, 5-fluorouracil (5-FU) and leucovorin in the treatment of advanced colorectal cancer.
Materials and Methods
Sixteen patients with relapsed or metastatic colorectal cancer were treated with an intravenous infusion of oxaliplatin 25 mg/m2, 5-FU 700 mg/m2 and leucovorin 20 mg/m2 on days 1 to 5. The infusion of oxaliplatin was chronomodulated with a peak delivery rate at 16:00 p.m., with 5-FU infused constantly overnight. Each course was repeated every 21 days.
Results
The response rate was 38.5% (95% confidence interval [CI], 13.9% to 68.4%) in the 13 measurable patients, including 1 complete response (7.7%) and 4 partial responses (30.8%). Five patients (38.5%) had a stable disease and 3 (23.0%) a progressive disease. Three patients without a measurable lesion had improved status. The median time to progression and overall survival were 29 weeks and 85 weeks, respectively. Grade 3 thrombocytopenia occurred in 2.5% (2 cycles) and grade 3 vomiting in 12.5% (2 patients). Anorexia, stomatitis, diarrhea, pruritus, alopecia and peripheral neuropathy were mild and tolerable.
More than 50% of patients with colorectal cancer have an incurable disease at the time of diagnosis, and are indicated for chemotherapy. Oxaliplatin and irinotecan, two newly developed agents with significant activity against colorectal cancer, have achieved relatively high response rates, but the median survival time has usually ranged from 9 to 12 months in patients who relapse following previous treatment with 5-FU and leucovorin (1). These results emphasize the need for new drugs and novel approaches in the treatment of metastatic colorectal cancer.
Chronotherapy consists of chemotherapy delivery according to biological rhythms along a 24-hour scale (2~6). These genetically based rhythms modulate the cellular metabolism and proliferation in normal tissues (7). As a result of chronotherapy, the tolerability and antitumor efficacy of 5-FU and oxaliplatin, among 30 anticancer drugs tested in laboratory rodents, varied largely according to the dosing time (8). Our aim was to transfer this concept to the clinic to primarily increase the dose-intensity. Specific technology (programmable-in-time injectors) allows for the administration of chronotherapy to fully ambulatory patients. Boughattas et al. administered lethal dose of oxaliplatin to 204 mice in one of three circadian stages (0, 8 or 16 hours after light onset), and found the toxicity was less severe at 16 hours (8). These results showed that administration of oxaliplatin during the daytime, especially 16:00 p.m., decreased the toxicities. The activity of dehydropyrimidine dehydrogenase increased around midnight and the tolerance of 5-FU is improved from 00:00 hours to 04:00 hours (9,10).
Fluorouracil (5-FU) has only achieved 10% of the objective responses in colorectal cancer, when given as a single agent for the first-line treatment of a metastatic disease (11). The objective response rate was increased by combining 5-FU with leucovorin (LV), as biochemical modulators (11,12), or by administrating 5-FU as a continuous venous infusion (11,13,14).
Oxaliplatin is a diaminocyclohexane platinum complex. Similarly to cisplatin and carboplatin, its main mechanism of action is mediated by the formation of DNA adducts. Oxaliplatin displays in vitro activity against human colorectal cancer cells (15), and has exhibited in vivo synergistic antitumor activity with 5-FU against transplantable tumor models (16,17). When used as a single agent, oxaliplatin achieved a 10~24% objective response rate in metastatic colorectal cancer (2,18~20).
The chronotherapy of oxaliplatin, 5-FU and leucovorin has achieved 51~67% objective responses and shown good tolerability in previously untreated metastatic colorectal cancer (3~6). In two phase III trials, chronotherapy reduced the toxicities and significantly increased the objective response rate (51~53%) compared with a constant-rate infusion of oxaliplatin (29~32%)(5,6).
Herein, the efficacy and toxicity of chronotherapy of oxaliplatin, 5-FU and leucovorin has been investigated in patients with advanced colorectal cancer.
Eligible patients had a histologically or cytologically confirmed adenocarcinoma of the colon or rectum, with relapsed or progressive disease patients, after 5-FU based chemotherapy, and metastatic lesions were included. Other eligibility criteria were measurable or evaluable lesions of tumor, an age between 18 and 75 years, a performance status by the ECOG scale, of grades 0 or 1, adequate organ function and a life expectancy of at least 3 months. Pretreated patients had received 5-FU based regimens as adjuvant or palliative treatments.
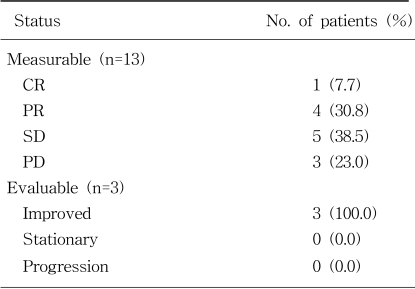
Pretreatment evaluation included: a complete medical history and physical examination, complete blood cell counts, chemistry profile, CEA measurement, urine analysis, chest X-ray, and CT scans of the abdomen, pelvis and/or chest. A complete blood cell count was obtained before the start of each treatment cycle, together with a serum chemistry profile, CEA measurement, chest X-ray, physical examination and toxicity assessment. Patients had radiological tumor parameter assessments every two cycles. The tumor response classification was derived from standard WHO criteria: (1) complete response, complete disappearance of all symptoms and signs of disease for a minimum of 4 weeks; (2) partial response, a 50% reduction (or more) in the sum of the products of the perpendicular diameters of measurable disease and the appearance of no new malignant lesion for a minimum of 4 weeks; (3) stable disease, no appearance of new areas of disease or less than 50% decrease or less than 25% increase in the described measurements; and (4) progressive disease, more than 25% increase in the measurements and/or appearance of new lesions. Patients who had evaluable lesions only were classified as having improved, stationary and progressive status according to the CEA and radiological imaging studies. The response based on the CEA assessment was defined as a morethan 50% drop in the serum CEA level for more than four weeks (21).
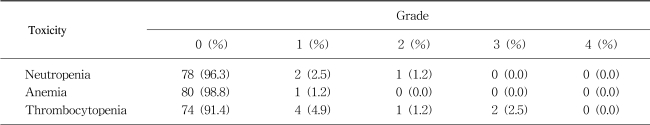
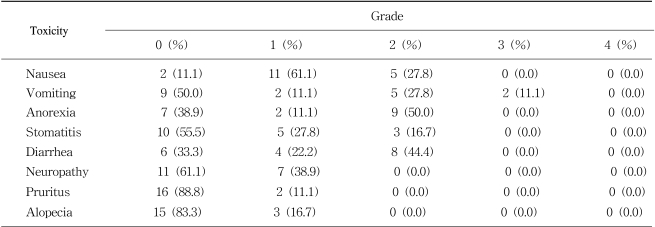
The toxicities of each course were recorded prior to the commencement of the subsequent course, and were graded according to the WHO criteria for neutropenia, anemia, thrombocytopenia, alopecia, nausea, vomiting, diarrhea, mucositis and peripheral sensory neuropathy.
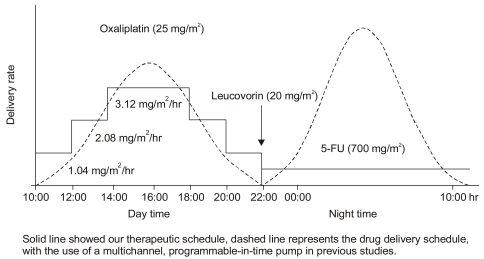
Patients received a 5-day course of chronomodulated, intravenously infused oxaliplatin (25 mg/m2/d), from 10:00 hours to 22:00 hours, with a continuous infusion of 5-FU (700 mg/m2/d) following a leucovorin bolus infusion (20 mg/m2/d) from 22:00 hours to 10:00 hours. This 5-day schedule (OHP-FL) was repeated every 3 weeks. Because a programmable-in-time, multichannel ambulatory pump for chronotherapy, with a sinusoidally varying delivery rate and peak delivery at 16:00 p.m., was not available, the authors modified the infusion rate using a stepladder configuration (Fig. 1).
Dose modifications were based on the complete blood cell counts taken the day before the next planned treatment. In case of grade 3 or 4 non-hematologic or grade 4 hematologic toxicities in the previous cycle, the oxaliplatin was reduced by 25% in the subsequent cycles, and the next course was delayed until complete recovery. The dose modification of 5-FU was performed as above.
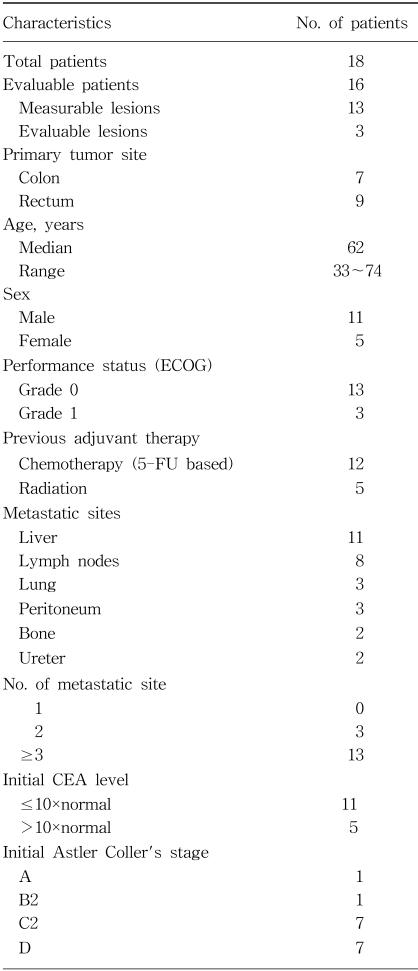
From March 1999 until December 2001, a total of 18 patients were enrolled. Two of these patients were excluded as they refused treatment after 1 cycle. Thirteen patients had measurable lesions and 3 did not, but all lesions were evaluable. Table 1 lists the demographic data, baseline disease and pretreatment characteristics for all patients. The median age of the patients was 62 years, ranging from 33 to 74. There were 11 male and 5 female patients. Seven and 9 patients had colon and rectal cancers, respectively. The most common disease sites were the liver (11 patients) and lymph node (8 patients).
Twelve patients had previously received chemotherapy containing 5-FU. Nine of the 12 previously-treated patients received chemotherapy as an adjuvant therapy. A relapse occurred after more than 6 months of adjuvant chemotherapy in 6 of these 9 patients. Fourteen patients had received surgery to remove the primary tumor, with 5 rectal cancer patients having received postoperative irradiation. Only 4 patients received OHP-FL chronotherapy as a first-line therapy with no other chemotherapy or radiotherapy. Of the 7 patients with colon cancer, 6 received a curative or palliative resection, and 4 received adjuvant chemotherapy. Of the 9 patients with rectal cancer, 8 received surgery, 5 of whom received adjuvant radiotherapy and chemotherapy, including 5-FU.
A total of 81 cycles of OHP-FL chemotherapy were performed (median: 5.5 cycles, range: 3~6 cycles). Two patients with ureteral metastasis had good renal function and did not required reduce chemotherapy. The mean dose-intensity (DI) of oxaliplatin per course was 34.8 mg/m2/week/course, which was 16.5% lower than that initially planned. The mean DI of 5-FU per course was 916.2 mg/m2/week/course, which was 22.5% lower than that initially planned. The median interval between the first days of two consecutive courses was 23 days (range, 19~36 days). Treatment was delayed for a median of 7 days for 18 (22%) of the eighty-one cycles.
The response rate was 38.5% (95% confidence interval [CI], 13.9% to 68.4%) for the 13 measurable patients, including 1 CR (7.7%) and 4 PR (30.8%). Five (38.5%) had stable disease and 3 (23.0%) progressive disease (Table 2). The response rate by intent-to-treatment was 33.4%, including 6.7% CR and 26.7% PR. Of the 4 patients who received OHP-FL chronotherapy as the first-line chemotherapy, without previous 5-FU based chemotherapy or radiotherapy, 3 showed a PR and the other a progressive disease. Of the 12 patients previously treated with 5-FU based chemotherapy, 1 showed CR, 1 PR, 5 stable disease (SD), 2 progressive disease (PD) and 3 improved status. Of the nine relapsed patients after the adjuvant chemotherapy 1 showed PR, 5 SD, 1 PD and 2 improved status. The three patients without a measurable lesion had an improved status. The serum CEA level was observed to drop morethan 50% and the small peritoneal lymph nodes (less than 1 cm) were decreased in all 3 patients.
Two patients showed complete disappearance of liver metastasis after the OHP-FL chronotherapy. One patient was assessed as CR who had initially been diagnosed as Astler-Coller stage D of rectosigmoid cancer, with multiple metastatic liver masses. He had received radiotherapy and chemotherapy with 5-FU and leucovorin, and then palliative surgery for resection of the primary rectosigmoid cancer due to obstruction symptoms. After 6 cycles of OHP-FL chronotherapy, the metastatic liver masses had all disappeared on the serial follow up CT finding, but had progressed 29 weeks later. Another rectal cancer patient, assessed as PR, with 3 metastatic masses in the liver, had received 4 cycles of OHP-FL chronotherapy. The metastatic masses of the liver completely disappeared. He received surgery for resection of the primary rectal cancer, with the result of the pathology revealing stage B2, with no recurrence until recently.
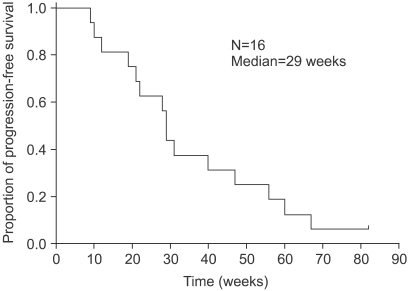
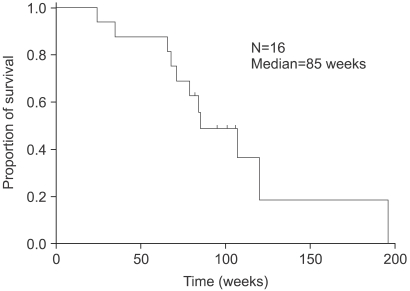
The median follow-up duration, time to progression (TTP) and overall survival were 85 (range, 24.0~196.0 weeks), 29 (Fig. 2) and 85 weeks (Fig. 3), respectively. The TTP and overall survival between the first- and second-line OHP-FL chemotherapy was not significantly different (p=0.45, p=0.61). With 2 subgroups of second-line OHP-FL chemotherapy, the TTP and overall survival of relapsed patients, compared with those of metastatic patients, were not statistically different (p=0.30, p=0.18). The survival rate of liver metastasis with lymph node involvement, compared with that of other metastatic sites, was not statistically different (p=0.10). Of the 16 patients, 11 died.
The incidences of hematological and non-hematological toxicities are summarized in Tables 3 and 4, respectively. There was 2.5% grade 3 thrombocytopenia (2 cycles), and 11.1% of grade 3 vomiting (2 patients). Anorexia, stomatitis, diarrhea, pruritus, alopecia and peripheral neuropathy were mild and tolerable (Table 4). No treatment-related mortality, infection and neutropenic fever occurred. No hand-foot syndrome developed.
The results of this study have shown that the efficacy of a chronomodulated infusion of oxaliplatin, 5-FU and leucovorin are comparable with the constant-rate infusion, FOLFOX regimen. The response rate was 38.5% (1 CR and 4 PR), and 1 of partial responders who had rectal cancer with 3 metastatic masses in the liver, who had received 4 cycles of chemotherapy. The metastatic masses of the liver completely disappeared. He received surgery for resection of the primary rectal cancer, with the pathology results revealing stage B2. FOLFOX studies have shown relatively lower response rates, of between 20 and 36%, with the exception of FOLFOX2 (46%)(22~25).
The role of chronotherapy has been investigated in several trials. In phase II trials, a chronomodulated infusion of oxaliplatin achieved a relatively high objective response rate (58~67%) and complete response rate (3~11%), with good tolerability in patients previously untreated for metastatic colorectal cancer (3,4). In phase III trials, chronotherapy reduced the incidences of severe toxicities (grade 3 or 4), while significantly increasing the objective response rate (51~53%) compared with the constant-rate delivery oxaliplatin schedules (29~32%)(5,6).
In this study, the objective response rate was lower than those of previous trials. One of the possible explanations is that all 3 drugs were chronomodulately infused in these trials, while only the oxaliplatin was chronomodulately infused in this study. A second could be the small size of enrolled patients, as this study was only a pilot. A third explanation could be that the patients in this study had more advanced stages, with 2 or more metastatic sites, than those in previous studies, with those enrolled almost receiving the treatment as a second-line chemotherapy.
In this trial, the oxaliplatin was infused step by step, with a peak delivery rate at 16:00 hours, with the 5-FU infused at a constant rate overnight (Fig. 1). This schedule was devised to avoid using the programmable-in-time injectors, with the convenience of an overnight infusion. Most chronomodulated infusion trials have been designed to infuse 5-FU from 22:00 hours to 10:00 hours, with a peak delivery rate at 04:00, with oxaliplatin from 10:00 hours to 22:00 with the peak delivery rate at 16:00 hours (3~6). Our modified infusion schedule may affect the efficacy and toxicity of this three-drug regimen.
Besides the efficacy, the toxicity of this regimen was tolerable. There was 2.5% grade 3 thrombocytopenia (2 cycles), and 11.1% grade 3 vomiting (2 patients). In previous chronomodulated infusion trials, grade 3 or 4 neutropenia occurred with 0.1~1.8% of the courses, and grade 3 or 4 thrombocytopenia with 0.1~0.4% of the courses (3,4). Grade 3 or 4 nausea and vomiting occurred in 24~37.8% of patients (4~6). Grade 3 or 4 diarrhea did not occur in our study, but frequently occurred in 24~41% of the patients in previous trials (4~6).
Peripheral sensory neuropathy was the cumulative dose-limiting toxicity. Seven patients complained of grade 1 neurotoxicity. In previous trials, grade 2 peripheral sensory neuropathy occurred in 25.6~27% of the patients, but no grade 3 or 4 neuropathy occurred (4,5). In another report, grade 1 or 2 neuropathy occurred in 453 courses (58%), while grade 3 or 4 neuropathy occurred in 86 courses (11%), which eventually led to oxaliplatin withdrawal in 14 patients after 7~12 courses, with complete recovery within 3 months (3).
References
1. Rougier P, Van Cutsem E, Bajetta E, Niederle N, Possinger K, Labianca R, Navarro M, Morant R, Bleiberg H, Wils J, Awad L, Herait P, Jacques C. Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet. 1998; 352:1407–1412. PMID: 9807986.

2. Levi F, Perpoint B, Garufi C, Focan C, Chollet P, Depres-Brummer P, Zidani R, Brienza S, Itzhaki M, Iacobelli S, Kunstlinger F, Gastiaburu J, Misset JL. Oxaliplatin activity against metastatic colorectal cancer: A phase II study of 5-day continuous venous infusion at circadian rhythm modulated rate. Eur J Cancer. 1993; 29:1280–1284. PMID: 8343268.

3. Levi F, Misset JL, Brienza S, Adam R, Metzger G, Itzakhi M, Caussanel JP, Kunstlinger F, Lecouturier S, Descorps-Declere A, Jasmin C, Bismuth H, Reinberg A. A chronopharmacologic phase II clinical trial with 5-fluorouracil, folinic acid, and oxaliplatin using an ambulatory multichannel programmable pump: High antitumor effectiveness against metastatic colorectal cancer. Cancer. 1992; 69:893–900. PMID: 1735081.

4. Levi F, Zidani R, Brienza S, Dogliotti L, Perpoint B, Rotarski M, Letourneau Y, Llory JF, Chollet P, Rol AL, Focan C. A multicenter evaluation of intensified, ambulatory, chronomodulated chemotherapy with oxaliplatin, 5-fluorouracil, and Leucovorin as initial treatment of patients with metastatic colorectal carcinoma. Cancer. 1999; 85:2532–2540. PMID: 10375099.

5. Levi F, Zidani R, Vannetzel JM, Perpoint B, Focan C, Faggiuolo R, Chollet P, Garufi C, Itzhaki M, Dogliotti L, Iacobelli S, Adam R, Kunstlinger F, Gastiaburu J, Bismuth H, Jasmin C, Misset JL. Chronomodulated versus fixed-infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid in patients with colorectal cancer metastases. A randomized multi-institutional trial. J Natl Cancer Inst. 1994; 86:1608–1617. PMID: 7932825.
6. Levi F, Zidani R, Misset JL. Randomised multicentred trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. For the International Organization for Cancer Chronotherapy. Lancet. 1997; 350:681–686. PMID: 9291901.
7. Antoch MP, Song EJ, Chang AM, Vitaterna MH, Zhao Y, Wilsbacher LD, Sangoram AM, King DP, Pinto LH, Takahashi JS. Functional identification of the mouse circadian Clock gene by transgenic BAC rescue. Cell. 1997; 89:655–667. PMID: 9160756.

8. Boughattas NA, Levi F, Fournier C, Lemaigre G, Roulon A, Hecquet B, Mathe G, Reinberg A. Circadian rhythm in toxicities and tissue uptake of 1,2-diamminocyclohexane (trans-1) oxalatoplatinum (II) in mice. Cancer Res. 1989; 49:3362–3368. PMID: 2720689.
9. Harris BE, Song R, Soong S, Diasio RB. Relationship between dihydropyrimidine dehydrogenase activity and plasma 5-fluorouracil levels with evidence for circadian variation of enzyme activity and plasma drug levels in cancer patients receiving 5-fluorouracil by protracted continuous infusion. Cancer Res. 1990; 50:197–201. PMID: 2293556.
10. Zhang R, Lu Z, Liu T, Soong SJ, Diasio RB. Relationship between circadian-dependent toxicity of 5-fluorodeoxyuridine and circadian rhythms of pyrimidine enzymes: possible relevance to fluoropyrimidine chemotherapy. Cancer Res. 1993; 53:2816–2822. PMID: 8504424.
11. Giacchetti S, Perpoint B, Zidani R, Bail NL, Faggiuolo R, Focan C, Chollet P, Llory JF, Letourneau Y, Coudert B, Bertheaut-Cvitkovic F, Larregain-Fournier D, Rol AL, Walter S, Adam R, Misset JL, Levi F. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000; 18:136–147. PMID: 10623704.

12. Advanced Colorectal Cancer Meta-Analysis Project: Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: Evidence in terms of response rate. J Clin Oncol. 1992; 10:896–903. PMID: 1534121.
13. Lokich JJ, Ahlgren JD, Gullo JJ, Philips JA, Fryer JG. Prospective randomized comparison of continuous infusion of fluorouracil with a conventional bolus schedule in metastatic colorectal carcinoma: A Mid-Atlantic Oncology Program study. J Clin Oncol. 1989; 7:425–432. PMID: 2926468.
14. Meta-Analysis Group in Cancer: Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol. 1998; 16:301–308. PMID: 9440757.
15. Pendyala L, Creaven PJ. In vitro cytotoxicity, protein binding, red blood cell partitioning, and biotransformation of oxaliplatin. Cancer Res. 1993; 53:5970–5976. PMID: 8261411.
16. Tashiro T, Kawada Y, Sakurai Y, Kidani Y. Antitumor activity of a new platinum complex, oxalato (trans-1-1,2-diaminocyclohexane) platinum (II): New experimental data. Biomed Pharmacother. 1989; 43:251–260. PMID: 2790145.
17. Mathe G, Kidani Y, Segiguchi M, Eriguchi M, Fredj G, Peytavin G, Misset JL, Brienza S, de Vassals F, Chenu E, Bourutet C. Oxalato-platinum or I-OHP, a third generation platinum complex: An experimental and clinical appraisal and preliminary comparison with cisplatinum and carboplatinum. Biomed Pharmacother. 1989; 43:237–250. PMID: 2675999.
18. Machover D, Diaz-Rubio E, de Gramont A, Schilf A, Gastiaburu JJ, Brienza S, Itzhaki M, Metzger G, N'Daw D, Vignoud J, Abad A, Francois E, Gamelin E, Marty M, Sastre J, Seitz JF, Ychou M. Two consecutive phase II studies of oxaliplatin (L-OHP) for treatment of patients with advanced colorectal carcinoma who were resistant to previous treatment with fluoropyrimidines. Ann Oncol. 1996; 7:95–98. PMID: 9081400.

19. Diaz-Rubio E, Sastre J, Zaniboni A, Labianca R, Cortes-Funes H, de Braud F, Boni C, Benavides M, Dallavalle G, Homerin M. Oxaliplatin as a single agent in previously untreated colorectal carcinoma patients: A phase II multicentric study. Ann Oncol. 1998; 9:105–108. PMID: 9541691.
20. Becouarn Y, Ychou M, Ducreux M, Borel C, Bertheault-Cvitkovic F, Seitz JF, Nasca S, Nguyen TD, Paillot B, Raoul JL, Duffour J, Fandi A, Dupont-Andre G, Rougier P. Phase II trial of oxaliplatin as first-line chemotherapy in metastatic colorectal cancer patients. J Clin Oncol. 1998; 16:2739–2744. PMID: 9704726.
21. Wang WS, Lin JK, Lin TC, Chiou TJ, Liu JH, Yen CC, Chen WS, Jiang JK, Yang SH, Wang HS, Chen PM. Tumor marker CEA in monitoring of response to tegafur-uracil and folinic acid in patients with metastatic colorectal cancer. Hepatogastroenterology. 2002; 49:388–392. PMID: 11995458.
22. Bae YZ, Jung JH, Moon CH, Kim SH, Kwon HC, Kim JS, Kim HJ. A phase II study of oxaliplatin combined with 5-fluorouracil and leucovorin (Mayo Clinic Regimen) in 5-fluorouracil refractory colorectal cancer. Cancer Res Treat. 2002; 34:218–222.

23. Maindrault-Goebel F, Louvet C, Andre T, Carola E, Lotz JP, Molitor JL, Garcia ML, Gilles-Amar V, Izrael V, Krulik M, de Gramont A. Oxaliplatin added to the simplified bimonthly leucovorin and 5-fluorouracil regimen as second-line therapy for metastatic colorectal cancer (FOLFOX6). Eur J Cancer. 1999; 35:1338–1342. PMID: 10658524.

24. Andre T, Louvet C, Raymond E, Tournigand C, de Gramont A. Bimonthly high-dose leucovorin, 5-fluorouracil infusion and oxaliplatin (FOLFOX3) for metastatic colorectal cancer resistant to the same leucovorin and 5-fluorouracil regimen. Ann Oncol. 1998; 9:1251–1253. PMID: 9862058.
25. Andre T, Bensmaine MA, Louvet C, Francois E, Lucas V, Desseigne F, Beerblock K, Bouche O, Carola E, Merrouche Y, Morvan F, Dupont-Andre G, de Gramont A. Multicenter phase II study of bimonthly high-dose leucovorin, fluorouracil infusion, and oxaliplatin for metastatic colorectal cancer resistant to the same leucovorin and fluorouracil regimen. J Clin Oncol. 1999; 17:3560–3568. PMID: 10550155.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download