Abstract
Purpose
c-myc, bcl-2 and p53 are known to regulate apoptosis. There has been growing interest in analyzing their contribution to the pathogenesis and prognosis in a variety of human cancers. This study was undertaken to investigate the expression of these proteins in pulmonary adenocarcinomas and to determine their relationship with clinicopathologic parameters and survival.
Materials and Methods
Archival tumor tissues from 61 patients with adenocarcinoma of lung were analyzed by immunohistochemistry for the expression of c-myc, bcl-2 and p53 proteins. Clinical information was obtained through the computerized retrospect database from the tumor registry.
Results
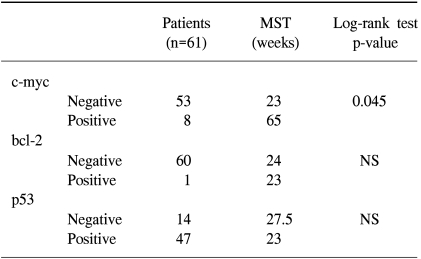
Of 61 patients, 32 were men and 29 women with the median age 63 years. 4 had stage I disease, 2 had stage II disease and 55 had stage III disease. The expression of c-myc protein was identified in 13% (8/61) tumors, bcl-2 protein was detected in 1.6% tumors (1/61) and p53 was detected in 77% (47/71) tumors. The association of the expression of c-myc, bcl-2 and p53 was not detected. The survival time was longer in patients expressing c-myc protein than in patients without the c-myc protein expression (p=.045). Neither bcl-2 nor p53 showed the correlation to clinicopathologic variables.
Conclusion
Our data suggest the involvement of p53 alteration in the pathogenesis of lung adenocarcinoma. The c-myc expression in some tumors indicates that c-myc alone may not contribute critically to the development and/or the progression of these tumors. It, however, correlated to the survival time, suggesting the c-myc expression as a favorable prognostic factor possibly through the apoptosis pathway.
Apoptosis is a distinct mode of cell death that is responsible for the deletion of cells in embryogenesis, the normal turnover of adult tissues and of malignant tumors (1). Oncogenes and tumor suppressor genes have been reported to play a role in the apoptosis pathway (2). It is well-known that c-myc, bcl-2 and p53 regulate apoptosis. Increased c-myc expression has been shown to induce mitosis or apoptosis, depending on the availability of other critical growth stimuli. In the presence of such stimuli, c-myc acts as a classic proto-oncogene stimulating mitosis; in the absence of such stimuli, it initiates apoptosis (1). The dysregulated expression of c-myc contributes to the tumorigenesis of cervix, breast and colon cancer. Thus, there has been growing interest in analyzing its contribution to the pathogenesis and prognosis of lung cancer (3~7). The tumor suppressor function of wild-type p53 is based on its capacity to produce G1 arrest and direct cells toward apoptosis whereas mutated p53 loses its capacity to induce apoptosis (8,9). A relationship between the p53 abnormality and the c-myc activation has been suggested (10,11). The bcl-2 protein is a known inhibitor of the p53-dependent and independent apoptosis pathway. Bcl-2 at high concentrations protects cells apoptosis induced by c-myc or wild-type p53 (12~14). However, the associations of these proteins and the correlation of their expression and the prognosis of lung cancer are still controversial (6,7,15~17).
Lung cancer is a major cause of cancer death worldwide. Lung cancer has become the leading cause of cancer death in Korea. Although recent molecular studies have provided increased understanding of the biology of lung cancer, the essential genetic features along with factors for prognosis are yet to be determined. Presently, most studies focused on non-small cell lung cancer (NSCLC) including adenocarcinomas without analyzing them separately. Thus, the literature contains limited data regarding these proteins in pulmonary adenocarcinoma, which may have a different mechanism of tumorigenesis as compared to other types of NSCLC. Furthermore, in many parts of the world, the incidence of adenocarcinoma of the lung has shown to be increased, possibly due to the increasing number of women who smoke. Women appear to be predisposed to develop carcinoma of this particular histological pattern (18). Another contributing factor may be the increasing popularity of low-tar and filter-tipped cigarettes.
Here, we evaluated the expression of c-myc, bcl-2 and p53 proteins in adenocarcinoma of the lung by staining formalin-fixed, paraffin-embedded, lung tumor tissue from patients with adenocarcinoma with corresponding antibody. Subsequently, the correlation of the expression of c-myc, bcl-2 or p53 proteins and clinicopathologic parameters was analyzed.
61 primary tumor tissues were obtained from patients with pulmonary adenocarcinoma. All patients were surgically treated at the Catholic University, St Vincent's Hospital between January 1, 2001 and December 31, 2002. None of patients had received therapy previously. Tissue samples were fixed in 10 percent buffered formalin. After routine embedding, the final diagnosis was made by the light microscope examination. One author (JY) reviewed the histopathologic diagnosis and the tumor type according to the relevant WHO classification, tumor grade and the quality of tissue sections. Clinical information was obtained through the computerized retrospective database of tumor registry. Follow-up data were available from 61 patients for the duration ranging from 4 to 138 weeks (31 months), with the mean 78 weeks (18 months). 24 patients died during follow-up and 37 were alive at the time of study.
For the detection of c-myc, bcl-2 and p53, immunohistochemical staining was performed on the representative sections of archival, formalin-fixed, paraffin-embedded materials by the peroxidase-streptavidin method as described previously (19). Briefly, each block was cut into 5 µm thick sections, which were deparaffinized in xylene and rehydrated in graded alcohols and water. Endogenous peroxidase was blocked by incubating in 3% H2O2 at 45℃ for 4 minutes. The slides were heated in citrate buffer (2.1 g/l, pH 6.0) at 120℃ for 15 minutes to unmask the antigen, treated with a protein blocking reagent, and incubated at 4℃ overnight with primary antibody at 1:50 dilution as recommended by the supplier. The primary antibodies used were anti-c-myc antibody (NeoMarkers, Fremont, CA), anti-bcl-2 antibody (Zymed Laboratories Inc., San Francisco, CA) and anti-p53 antibody (Zymed). After extensive washing, the sections were incubated at room temperature for 10 minutes with biotinylated anti-mouse immunoglobulin antibodies (Zymed) at 1:20 dilution and subsequently with streptavidin-biotin peroxidase complexes at 1:25 dilution. The staining was developed with 3-amino-9-ethylcarbazole. The nuclei were counterstained with Meyer's hematoxylin.
All staining included positive and negative controls. The positive control for the expression of c-myc and p53 protein was breast carcinoma and for the expression of bcl-2 protein was tonsil. As the negative control of the immunohistochemistry technique, the incubation with primary antibody was omitted. Tumor cells with nuclear staining were considered as positive for the c-myc and p53 expression. Tumor cells with cytoplasmic staining was considered as positive for the bcl-2 protein. Three investigators examined the section staining and obtained the identical results in 92% cases. The remaining samples reviewed jointly and the consensus was obtained.
Statistical analysis was performed using the software SPSS 10.0 (Seoul, Korea). Two-sided P value was determined by the log-rank test. The level of significance was set at 0.05. Survival was measured in weeks from the date of surgery. The actuarial survival curve was plotted with the method of Kaplan and Meier. The data other than survival were analyzed using Fisher's exact test.
Clinical data are shown in Table 1. 61 patients, 32 men and 29 women, were recruited. Their mean age was 61 years, ranged from 34 to 87 years. Patients younger than 61 years had significantly longer survival (p=0.018). However, the correlation of the patient's age and tumor extent, lymph node involvement or tumor stage was not detected (Table 2). A possible explanations for the better prognosis in younger patients may be the rapid and uncomplicated recovery after surgery. All patients were staged at the time of their surgery according to the guidelines of the American Joint Committee on Cancer. Four had stage I disease, 2 had stage II disease, and 55 had stage III disease. The tumor extent had no effect on survival: the mean survival in patients with T1 or T2 tumors was 25 weeks and in patients with T3 or T4 tumors was 27 weeks. On the other hand, the median survival time in patients without the lymph node involvement was 28 weeks. The median survival time in patients with the lymph node involvement was decreased to 17 weeks. The median survival was longer in patients with stage I or II tumors than in patients with stage III tumors. Although the difference was not statistically significant, stage and lymph node involvement may have effect on survival.
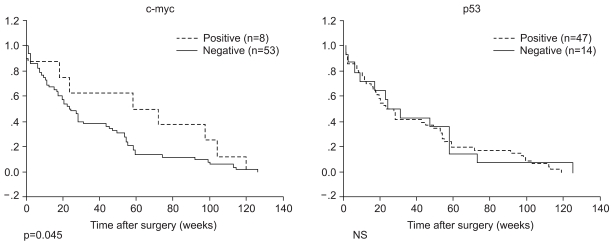
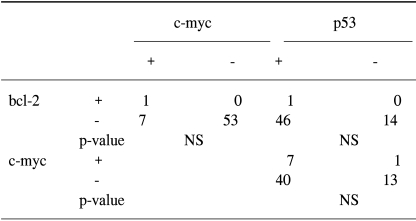
The c-myc, bcl-2 and p53 protein was examined in 61 tumor samples. Negative and positive controls showed the expected results. The results of the analysis are summarized in Table 3. The expression of c-myc protein was identified in 13% samples (8/61) (Fig. 1A). 7% (3/42) survived less than 1 year. 26% (5/19) survived for 1 year or longer (p=.041). The bcl-2 protein was detected in only 1 patient. The overexpression of p53 protein was detected in 77% tumor samples (47/61) (Fig. 1B). The mean survival time in patients with tumors expressing the c-myc protein was significantly longer than in patients with tumors absent the c-myc protein (65 weeks vs. 23 weeks, p=0.045). The association of the expression of c-myc, bcl-2 and p53 was not detected (Table 4). Other clinicopathologic parameters had no significant correlation to the expression of c-myc or p53 protein. Fig. 2 shows the survival curve of patients according to their expression of the c-myc and p53 protein estimated by Kaplan-Meier method.
Here, we analyzed the correlation of the expression of the protooncogenes,c-myc and bcl-2, and the suppressor gene product p53 in primary pulmonary adenocarcinomas to clinical parameters such as patient's age, sex, the tumor extent, the presence of lymph node metastasis, tumor stage and patient survival. 61 samples were analyzed.) Our data demonstrate that: (1) the c-myc protein was detected in a small number of tumor specimens (13/61). The expression, however, correlates to survival, (2) the bcl-2 oncoprotein was detected in only one case, (3) the altered p53 protein was detected in the majority of the samples (47/61).
The understanding of lung cancer pathogenesis is rapidly growing. The evidence indicates that several molecular genetic markers are involved in the initiation and/or progression of the disease. However, the interaction of the molecular genetic changes is complex and some of which are still controversial in predicting aggressive behavior. The c-myc oncogene is known to regulate neoplastic development and apoptotic cell death (20). It has been reported to be amplified in small cell lung cancer (SCLC) and NSCLC, and has been linked to the malignancy of these cancers (3-7). The relatively high incidence of the c-myc amplification in tumor cell lines derived from SCLC than non-SCLC was detected (50~83% vs. 11~24%). This suggests that the myc family DNA amplification is not required for the initial steps of malignant transformation (5). Most investigations in the biology of lung cancer include either SCLC and/or NSCLC without histologic subtypes given in detail. However, squamous cell carcinomas and adenocarcinomas are morphologically quite different and differing genetic mechanisms may be involved. In this paper, we have focused on pulmonary adenocarcinomas and found that c-myc plays a role as a prognostic factor in these tumors. Presently, conflicting results have been obtained regarding the frequency and the clinical significance of the c-myc gene in different series of lung cancer, possibly due to the difference in tumor differentiation, histologic features, clinical stages, tumor grades and analytical procedures. The myc oncogens are usually found to be altered in SCLC, and the alteration appears to correlate to rapid growth and poor prognosis (3-5,21). The c-myc expression rate was reported to be higher in squamous cell lung carcinomas than in adenocarcinomas (49% vs. 29%) (7, 22). On the other hand, other investigators could not detect the statistically significant difference between groups of SCLC and NSCLC, among different histologic subtypes (squamous cell carcinomas, adenocarcinomas, large cell carcinomas and small cell carcinomas) of bronchial cancers in the prevalence of its expression, and the relationship between c-myc expression and other clinical parameters (6,7,22). Our observation of the association of the c-myc expression with survival is not surprising as the c-myc expression is well-known to be significantly enhanced in well-differentiated tumors (23). Six out of 8 tumors that we detected the expression of c-myc protein were well differentiated (data not shown). The clinical importance of the observations is supported by the reported correlation between the low expression of the c-myc gene product and poor prognosis (24). Further studies on the alteration of this gene in larger series along with long-term follow-up will clarify the role of c-myc protein in prognostic implication of pulmonary adenocarcinomas and may yield useful prognostic information in future.
The proto-oncogene bcl-2 encodes a protein that protects cells from apoptosis (25). It is known to be involved in the 14;18 translocation, a chromosomal abnormality present in a portion of human B-cell lymphomas (1). However, the data on the bcl-2 expression in lung cancer are very limited. Furthermore the prognostic significance of bcl-2 is controversial. Initially, the bcl-2 expression in NSCLC has been reported to be a favorable prognostic factor. Subsequent studies, however, did not support this result. Pezzella et al. has recently proposed that the bcl-2 expression may have prognostic importance in non-small cell lung carcinomas: they detected the bcl-2 protein in 20% of tumors and its expression correlated to survival (15). Gaffney et al. demonstrated that the bcl-2 expression in some lung carcinomas was not related to the extent of apoptosis and prognosis (16). In our study, the bcl-2 protein was present in only one sample. This is in agreement with the results of other investigations on pulmonary adenocarcinomas, the range was from 0 to 12% (14). The lack of bcl-2 expression suggests that bcl-2 protein does not play a critical role in the tumorigenesis of these tumors.
In contrast to bcl-2, the p53 alteration was identified in the majority of our samples, which may indicate their involvement in the pathogenesis of adenocarcinomas of the lung, although they did not provide additional prognostic information.
In this study, our data suggest that c-myc alone may not contribute critically to the development and/or progression of pulmonary adenocarcinomas. P53, on the other hand, may play an important role in the tumorigenesis of these tumors. It is noteworthy that c-myc may be a better prognostic factor. Our results show the absence of the correlation of the expression of c-myc, bcl-2 and p53, which suggests that there may be other oncogene products and/or additional factors that regulate apoptosis in vivo.
References
1. Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994; 73:2013–2026. PMID: 8156506.

2. Wyllie AH. Apoptosis. Br J Cancer. 1993; 67:205–208. PMID: 8431353.
3. Barr LF, Campbell SE, Diette GB, Gabrielson EW, Kim S, Shim H, Dang CV. c-myc suppresses the tumorigenicity of lung cancer cells and down-regulates vascular endothelial growth factor expression. Cancer Res. 2000; 60:143–149. PMID: 10646866.
4. Prins J, De Vries EG, Mulder NH. The myc family of oncogenes and their presence and importance in small-cell lung carcinoma and other tumor types. Anticancer Res. 1993; 13:1373–1385. PMID: 8239508.
5. Johnson BE, Ihde DC, Makuch RW, Gazdar AF, Carney DN, Oie H, Russell E, Nau MM, Minna JD. Myc family oncogene amplification in tumor cell lines established from small cell lung cancer patients and its relationship to clinical status and course. J Clin Invest. 1987; 79:1629–1634. PMID: 3034978.

6. Gosney JR, Field JK, Gosney MA, Lye MD, Spandidos DA, Butt SA. C-myc oncoprotein in bronchial carcinoma: expression in all major morphological types. Anticancer Res. 1990; 10:623–628. PMID: 2164348.
7. Volm M, Rittgen W, Drings P. Prognostic value of ERBB-1, VEGF, cyclin A, FOS, JUN, and MYC in patients with squamous cell carcinomas. Br J Cancer. 1998; 77:663–669. PMID: 9484827.
8. Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991; 352:345–347. PMID: 1852210.

9. Shaw P, Bovey R, Tardy S, Sahli R, Sordat B, Costa J. Induction of apoptosis by wild-type p53 in a human colon tumor-derived cell line. Proc Natl Acad Sci USA. 1992; 89:4495–4499. PMID: 1584781.

10. Ragimov N, Krauskopf A, Navot N, Rotter V, Oren M, Aloni Y. Wild-type but not mutant p53 can repress transcription inhibition in vitro by interfering with the binding of basal transcription factors to the TATA motif. Oncogene. 1993; 8:1183–1193. PMID: 8479742.
11. Reisman D, Elkind NB, Roy B, Beamon J, Rotter V. c-myc transactivates the p53 promoter through a required downstream CACGTG motif. Cell Growth Differ. 1993; 4:57–65. PMID: 8494784.
12. McDonnell TJ, Marin MC, Hsu B, Brisbay SM, McConnell K, Tu SM, Campbell ML, Rodriguez-Villanueva J. The bcl-2 oncogene: apoptosis and neoplasia. Radiat Res. 1993; 136:307–312. PMID: 8278571.

13. Bissonnette RP, Echeverri F, Mahboubi A, Green DR. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature. 1992; 359:552–554. PMID: 1406975.

14. O'Neill AJ, Staunton MJ, Gaffney EF. Apoptosis occurs independently of bcl-2 and p53 over-expression in non-small cell lung carcinoma. Histopathology. 1996; 29:45–50. PMID: 8818693.
15. Pezzella F, Turley H, Kuzu I, Tungekar MF, Dunnill MS, Pierce CB, Harris A, Gatter KC, Mason DY. bcl-2 protein in non-small cell lung carcinoma. N Engl J Med. 1993; 329:690–694. PMID: 8393963.
16. Gaffney EF, O'Neil AJ, Staunton MJ. bcl-2 and prognosis in non-small cell lung carcinoma. N Engl J Med. 1994; 330:1757–1758. PMID: 8190145.
17. Anton RC, Brown RW, Younes M, Gondo MM, Stephenson MA, Cagle PT. Absence of prognostic significance of bcl-2 immunopositivity in non-small cell lung cancer: Analysis of 427 cases. Hum Pathol. 1997; 28:1079–1082. PMID: 9308733.

18. Corrin B, editor. Pathology of the lungs. 2000. London: Churchill Livingstone.
19. Yoo J, Kang SJ, Ahn WS, Kim BK. E-Cadherin expression and p53 alterations in soft tissue sarcomas: a possible role in epithelial differentiation. Cancer Res Treat. 2001; 33:343–349.

20. Spencer CA, Groudine M. Control of c-myc regulation in normal and neoplastic cells. Adv Cancer Res. 1991; 56:1–48. PMID: 2028839.

21. Slebos RJ, Rodenhuis S. The molecular genetics of human lung cancer. Eur Respir J. 1989; 2:461–469. PMID: 2547647.
22. Volm M, Efferth T, Mattern J. Oncoprotein (c-myc, c-erbB1, c-erbB2, c-fos) and suppressor gene product (p53) expression in squamous cell carcinomas of the lung. Clinical and biological correlations. Anticancer Res. 1992; 12:11–20. PMID: 1348920.
23. Sikora K, Chan S, Evan G, Gabra H, Markham N, Stewart J, Watson J. c-myc oncogene expression in colorectal cancer. Cancer. 1987; 59:1289–1295. PMID: 3545431.

24. Watson JV, Stewart J, Evan GI, Ritson A, Sikora K. The clinical significance of flow cytometric c-myc oncoprotein quantitation in testicular cancer. Br J Cancer. 1986; 53:331–337. PMID: 3964537.

25. Korsmeyer SJ. bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992; 80:879–886. PMID: 1498330.

Fig. 1
The expression of c-myc and p53 protein in pulmonary adenocarcinoma. (A) c-myc, (B) p53, Strong expression is present in the nucleus of tumor cells (magnification ×100).
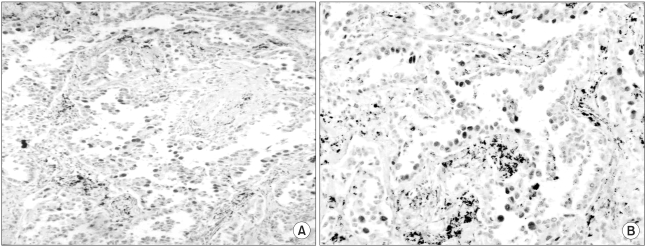
Fig. 2
The survival of pulmonary adenocarcinoma patients and the expression of c-myc and p53 protein.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download