Abstract
Purpose
The purpose of this study was to assess the efficacy and safety of radiofrequency ablation (RFA) to treat hepatic metastasis in patients with colorectal carcinoma.
Materials and Methods
Between May 1999 and July 2002, a total of 45 tumors in 24 patients with colorectal cancer were treated with RFA. Thirteen patients received systemic chemotherapy after the RFA procedure. The ablation was performed percutaneously under ultrasound guidance using cool-tip or expandable electrodes and an RF generator. The medical records as well as the CT scan results taken every 3 months were retrospectively reviewed.
Results
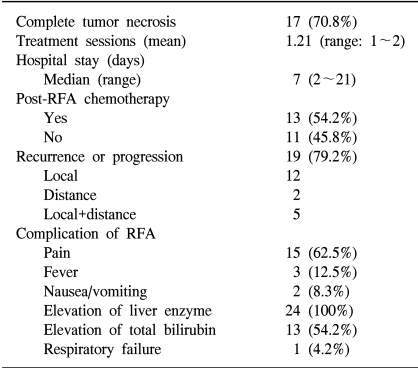
The median follow-up duration of the surviving patients was 11.7 months (4.6~32.2 months). Complete tumor necrosis was achieved in 17 patients (70.8%) on an immediate (<24 hrs) CT scan. The median survival was 17.1 months. The 1- and 2-year survival rates were 80.5 and 25.8%, respectively. In a univariate analysis, complete necrosis, tumor size and post-RFA chemotherapy were significant factors for survival. Nineteen of the 24 patients developed a recurrence or progressed (79.2%). The median progression free survival was 5.5 months. There were no treatment related deaths or serious adverse effects, with the exception of one case of respiratory failure.
The liver is a common site of distant metastasis in patients with colorectal carcinomas. Surgical resection of hepatic metastasis has been considered the only potentially curative treatment for patients whose metastases are isolated to the liver. Unfortunately, fewer than 20% of patients with liver only metastasis are eligible for curative resection (1~3). Therefore, novel treatment approaches to control and potentially cure liver disease are needed.
Radiofrequency ablation (RFA), a localized thermal treatment technique designed to produce tumor destruction by heating tumor tissue to temperatures exceeding 50℃, has been used as a treatment for primary and metastatic liver tumors, as well as a variety of other neoplasms, including osteoid osteoma, renal cell carcinoma, thyroid carcinoma, bone metastases and lung carcinoma (4~17). Ablative therapies based on the principle that decreasing the volume of a viable tumor, or preventing new growth, can lead to longer survival and potential cure in selected patients, provided a diffuse micrometastatic disease is not present. Currently, RFA is increasingly used to treat patients with inoperable colorectal liver metastases. This modality is considered safe and effective. However, the role of RFA still remains unclear.
The aim of this study was to describe the results of RFA in patients with hepatic metastasis of colorectal carcinomas.
Between May 1999 and July 2002, a total of 24 patients with colorectal cancer were treated with RFA for metastatic hepatic tumors at Hanyang University Hospital. Patients underwent a baseline evaluation, including a history, physical exam, CBC, blood chemistry, chest X-ray and CT scans of the abdomen and pelvis. The blood tests were repeated within 7 days of the RFA procedure. A CT scan was initially obtained within 1 day, and then every 3 months up to 2 years. The medical records of these patients were retrospectively reviewed. All patients signed written informed consent.
RFA was performed percutaneously with ultrasound-guidance under local and conscious sedation using lidocaine and demerol, respectively. All RFA procedures were performed by one radiologist (H Rhim). An expandable, 15-gauge, RF electrode (Monoploar 3D & StarBurst; RITA, MountainView, CA) and internally cooled, 18-gauge, RF electrodes (Cool-tip; Radionics, Burlington, MA) were used to apply RF energy to the tumors. The expandable electrodes were used for 18 patients during the early period, while the Cool-tip electrodes were used for 6, as from November 2001. RF ablation was performed with real-time US guidance using free-hand techniques. After cleaning the skin with iodized alcohol, the RF electrode was inserted into the tumor. Grounding was achieved with one or more grounding pads placed on the skin. The target volume for the ablation was defined as the entire tumor, with at least a 0.5 cm ablative margin of apparently normal hepatic tissue surrounding the tumor. For tumors larger than 3 cm in diameter, multiple overlapping ablations were often performed. After ablation, the electrode was removed without cauterization of the tract. Each ablation lasted for 7~15 minutes according to the device and target ablation volume. The mean total procedure time was 60 minutes per session, including tumor localization, treatment, electrode removal and immediate post-procedural US. A dynamic contrast-enhanced CT scan was performed within 12 hours of the procedure to assess the completeness of the ablation and to look for complications. If there was residual tumor on an immediate CT, repeat RF ablation was performed. Additional CT scans were obtained every 3 months after the procedure to look for evidence of residual or recurrent tumors. A systematic review of post therapeutic CT scans was performed to detect not only recurrence of tumor within the liver, but also for an extrahepatic malignancy.
The end points of this study were to evaluate the rates of recurrence and complications of RFA. The time to progression was defined as the time between the date of the RFA and the occurrence of disease progression. Survival was measured from the date of the RFA to the date of death or the last follow-up visit. Survival curves were estimated by the Kaplan-Meier method.
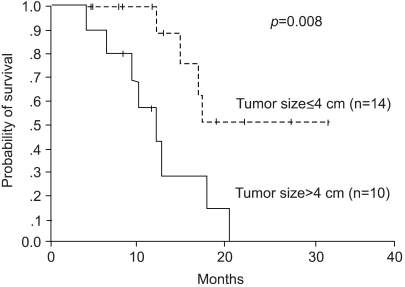
There were 13 male (54.2%) and 11 female (45.8%) patients, with a median age of 63.0 years, ranging from 29 to 77 years. A total of 45 metastatic hepatic tumors, in 24 patients with colorectal cancer, were treated with RFA. RFA was used to treat a single tumor in 11 (45.8%), two tumors in 7 (29.2%), three tumors in 4 (16.7%) and four tumors in 2 patients (8.3%). The median largest diameter of the tumors treated RFA was 3.4 cm (1.7~13.0 cm). 14 and 10 patients had tumors less than and larger than 4 cm, respectively.
All patients had undergone complete or palliative primary tumor resection prior to the initial RFA procedure. Four (16.7%) of the 24 patients underwent resection of liver metastases and 18 (75.0%) received systemic chemotherapy prior to the RFA. Thirteen patients received chemotherapy after the RFA. Eight patients were treated with an oxaliplatin based regimen (the median cycles: 6), two with an irinotecan based regimen and three received 5-fluorouracil.
The RFA results are summarized in Table 1. The rate of complete necrosis was significantly high in patients with tumor less than compared with those larger than 4 cm (p<0.05). The median progression free survival (PFS) and 1-year PFS were 5.5 months and 23.9%, respectively. The achievement of complete tumor necrosis was also an important factor for PFS (median PFS: 7.9 months vs. 3.0 months, p=0.048), but the number of metastasis or size of the tumor was not correlated to the PFS.
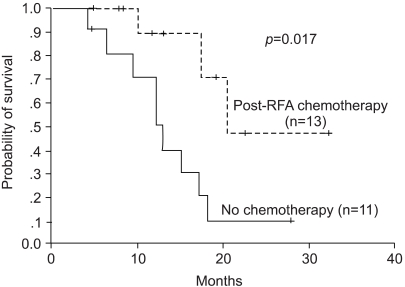
The median overall survival (OS) was 17.1 months. The 1- and 2-year survival rates were 80.5 and 25.8%, respectively. In the univariate analysis, complete tumor necrosis, tumor size and post-RFA chemotherapy proved significant factors for survival. The median survival of the patients with complete tumor necrosis was 17.5 months, whereas this was 12.8 months for the incomplete group (p=0.039). The median survival of the patients with tumor less than 4 cm was not reached, compared with the 12.2 months for those with tumors larger than 4 cm (p=0.008, Fig. 1). The patients who received post-RFA chemotherapy also had a significantly longer survival (median OS 20.5 months vs. 12.8 months, Fig. 2). However, the number of metastases did not influence the overall survival.
Twelve of the 24 patients died. The most common cause of death was disease progression, which occurred in 11 patients, and one patient died of complications due to chemotherapy.
Neither death nor serious adverse effects related to RFA were seen. Only 1 patient developed respiratory failure after RFA, but recovered completely with intensive medical care. The serum liver function tests (ALT and AST) were transiently elevated after RFA, but returned to baseline levels in most patients. The serum bilirubin levels rose in 13 patients (54.2%). Fever developed in 3 patients (12.5%), but no cases developed a hepatic abscess (Table 1).
Surgical resection remains the gold standard therapy for colorectal liver metastasis, but only a small portion of those patients are eligible for resection. Extrahepatic or multiple metastasis involving both lobes, and metastatic lesions in proximity to major blood vessels and the biliary structure preclude achieving an adequate margin. These patients may be candidates for local ablative techniques, hepatic-directed techniques or systemic chemotherapy.
Non-surgical, image-guided techniques for the ablation have recently generated much interest as they can be minimally invasive, easily repeated and safe. RFA is one of the most promising thermal ablation techniques. Since RFA was applied to liver tumors in the 1990s, its prevalence has rapidly increasing as an alternative or adjunctive to excision. However, the adequate role of RFA in the patients with metastatic colorectal cancer remains to be determined.
There have been a few studies on RFA in hepatic metastases from colorectal cancer. Solbiati et al (16) reported the results of RFA to treat 179 hepatic metastases in 117 patients with colorectal carcinomas. In the afore mentioned study, the median survival was 36 months and the 1, 2 and 3-year survival rates were 93, 69 and 46%, respectively, which were better than ours (median survival: 17.1 months, 1 and 2 year survival rates: 80.5 and 25.8%, respectively). The reasons for this discrepancy in the treatment outcomes may have been that our study showed a lower rate of complete necrosis of tumors compared with the study of Solbiati et al (70.8 vs. 100.0%). Generally, the rate of complete necrosis was related to tumor size. In our study, the rate of tumors larger than 4 cm was 41.7%, but of Solbiati's was only 10.6%. In addition, the results of Bleicher (18) also demonstrated that tumor size was the most significant factor in local recurrence. Although, there is no clear consensus regarding the size and number of lesions, most investigators consider 5 cm diameter tumors to be the maximum size that should be treated with RFA.
The clinical results of RFA for metastatic lesions are difficult to analyze for several reasons. First, most reported studies have involved different histologies and characteristics of the tumors. Second, different equipment and techniques are used for RFA. Third, many studies have combined RFA with other treatment modalities, such as an operation, chemotherapy and other techniques.
Currently, there is no standard therapeutic approach after a hepatic resection or RFA of colorectal metastasis. To treat suspected micrometastases in the remaining liver, and prevent extrahepatic spread, a reasonable approach involves the use of a combination of regional therapy, such as a hepatic arterial infusion (HAI) of chemotherapy, and systemic chemotherapy. Kemeny (19) investigated the effect of HAI with systemic chemotherapy after a hepatic metastatectomy. In this study, the use of HAI plus systemic chemotherapy not only decreased the rate of hepatic recurrence, but also improved the two-year overall survival compared with the use of systemic therapy alone. In another recently reported study (20), adjuvant systemic and HAI chemotherapy was compared with no adjuvant therapy following a hepatic metastatectomy, and the hepatic recurrence rate was significantly decreased in the chemotherapy arm. However, there was no significant difference in the median survivals. In our results, post-RFA chemotherapy was a significant prognostic factor for the overall survival. Although our results can not be used to determine the role of systemic chemotherapy after RFA, recently, new agents have been developed for colorectal cancer, promising results. It is our opinion that systemic chemotherapy after RFA may be needed.
This study suggested that RFA as a treatment for metastatic hepatic tumor in colorectal cancer was a safe, well-tolerated and effective modality. Although unlikely to be curative, RFA may be important for palliation and relief of symptoms and for improving the quality of life, in addition to increasing the survival in selected patients.
References
1. Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995; 19:59–71. PMID: 7740812.

2. Bismuth H, Adam R, Levi F, Farabos C, Waechter F, Castaing D, Majno P, Engerran L. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996; 224:509–520. PMID: 8857855.

3. Kim JC, Kim CN, Yu CS, Lee HI, Kim SW, Lee JH, Kim WK, Kang GH, Lee MK. Treatment of Hepatic Metastasis of Colorectal Cancer : A Retrospective Analysis of the Outcome in 99 Patients. J Korean Cancer Assoc. 1998; 30:1175–1183.
4. Rhim H, Goldberg SN, Dodd GD 3rd, Solbiati L, Lim HK, Tonolini M, Cho OK. Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. Radiographics. 2001; 21:S17–S35. PMID: 11598245.

5. Rosenthal DI, Springfield DS, Gebhardt MC, Rosenberg AE, Mankin HJ. Osteoid osteoma: percutaneous radio-frequency ablation. Radiology. 1995; 197:451–454. PMID: 7480692.

6. McGovern FJ, Wood BJ, Goldberg SN, Mueller PR. Radiofrequency ablation of renal cell carcinoma via image guided needle electrodes. J Urol. 1999; 161:599–600. PMID: 9915457.
7. Dupuy DE, Monchik JM, Decrea C, Pisharodi L. Radiofrequency ablation of regional recurrence from well-differentiated thyroid malignancy. Surgery. 2001; 130:971–977. PMID: 11742325.

8. Gronemeyer DH, Schirp S, Gevargez A. Image-guided radiofrequency ablation of spinal tumors: preliminary experience with an expandable array electrode. Cancer J. 2002; 8:33–39. PMID: 11898806.
9. Goldberg SN, Gazelle GS, Compton CC, Mueller PR, McLoud TC. Radio-frequency tissue ablation of VX2 tumor nodules in the rabbit lung. Acad Radiol. 1996; 3:929–935. PMID: 8959183.
10. Rossi S, Di Stasi M, Buscarini E, Quaretti P, Garbagnati F, Squassante L, Paties CT, Silverman DE, Buscarini L. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol. 1996; 167:759–768. PMID: 8751696.

11. Solbiati L, Goldberg SN, Ierace T, Livraghi T, Meloni F, Dellanoce M, Sironi S, Gazelle GS. Hepatic metastases: percutaneous radio-frequency ablation with cooled-tip electrodes. Radiology. 1997; 205:367–373. PMID: 9356616.

12. Jiao LR, Hansen PD, Havlik R, Mitry RR, Pignatelli M, Habib N. Clinical short-term results of radiofrequency ablation in primary and secondary liver tumors. Am J Surg. 1999; 177:303–306. PMID: 10326848.

13. Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, Fiore F, Pignata S, Daniele B, Cremona F. Radiofrequency ablation of unresectable primary and metastatic hepatic malignanies: results in 123 patients. Ann Surg. 1999; 230:1–8. PMID: 10400029.
14. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS. Hepatocellular carcinoma: radiofrequency ablation of medium and large lesions. Radiology. 2000; 214:761–768. PMID: 10715043.

15. Goldberg SN, Gazelle GS, Compton CC, Mueller PR, Tanabe KK. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer. 2000; 88:2452–2463. PMID: 10861420.
16. Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, Cova L, Halpern EF, Gazelle GS. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001; 221:159–166. PMID: 11568334.

17. Livraghi T, Solbiati L, Meloni F, Ierace T, Goldberg SN, Gazelle GS. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the "test-of-time approach". Cancer. 2003; 97:3027–3035. PMID: 12784338.
18. Bleicher RJ, Allegra DP, Nora DT, Wood TF, Foshag LJ, Bilchik AJ. Radiofrequency ablation in 447 complex unresectable liver tumors: lessons learned. Ann Surg Oncol. 2003; 10:52–58. PMID: 12513961.

19. Kemeny N, Huang Y, Cohen AM, Shi W, Conti JA, Brennan MF, Bertino JR, Turnbull AD, Sullivan D, Stockman J, Blumgart LH, Fong Y. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999; 341:2039–2048. PMID: 10615075.

20. Kemeny MM, Adak S, Gray B, Macdonald JS, Smith T, Lipsitz S, Sigurdson ER, O'Dwyer PJ, Benson AB 3rd. Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy--an intergroup study. J Clin Oncol. 2002; 20:1499–1505. PMID: 11896097.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download