Abstract
Purpose
The combination of chemoradiation and fluorouracil based chemotherapy has been the standard adjuvant treatment for colorectal cancer patients. The aim of this study was to evaluate treatment outcome of patients classified by the new AJCC staging system and to compare treatment outcome of oral doxifluridine and the standard Mayo Clinic regimen after chemoradiation in advanced rectal cancer patients.
Materials and Methods
One hundred nine patients underwent curative surgical resection and chemoradiation followed by chemotherapy. 45 Gy pelvic irradiation was given to the entire pelvis and the boost radiation with 50.4 to 54 Gy, and simultaneously 5-fluorouracil (5-FU) 375 mg/m2/day was given on day 1~3 and 26~28. After the completion of chemoradiation, patients were given either 6 cycles of the Mayo Clinic regimen (5-FU 425 mg/m2 plus leucovorin 20 mg/m2 intravenous bolus infusion on day 1~5, every 4 weeks) or oral doxifluridine (600 mg/m2/day) for 1 year.
Results
The median follow-up duration was 30 months. Among 102 evaluable patients, 38 patients (37.3%) relapsed: the locoregional recurrence in 10 patients (9.8%) and systemic relapse in 28 patients (27.5%). The systemic relapse rate was 15.6% in the stage IIA, 25.0% in the stage IIIB , and 59.1% in the stage IIIC (p=0.048). The 5-year disease-free survival (DFS) rate was significantly higher in the IIA and IIIA patients than the IIIB and IIIC patients (72% and 100% vs 48.1% and 11.2%, respectively. p<0.001). The 5-year overall survival (OS) rate was also significantly different between in the IIA/IIIA patients and the IIIB/IIIC (67.3%/100% vs 48.4%/22.3%. p<0.001). However, the difference in DFS or OS between the oral doxifluridine group and the Mayo Clinic regimen group was not significant. Cox regression multivariate analyses showed that the new AJCC stage and tumor differentiation were significant independent prognostic factors in DFS and OS.
Conclusion
These results support that the new AJCC staging system is superior to Dukes' staging system in the prognostic stratification. Regarding DFS and OS, oral doxifluridine is comparable to the standard Mayo Clinic regimen in rectal cancer patients when combined with postoperative chemoradiation. Stage IIIC patients should be selected for aggressive therapy as they have a dismal prognosis.
In the United States, colorectal cancer ranks as the third most common cancer (1). In Korea, colorectal cancer is the fourth most common cancer. The incidence of colorectal cancer in Korea is lower than in Western countries. Recently, the incidence of colorectal cancer in Koreans has increased recently (2).
Surgical resection is the cornerstone of curative therapy. Following a potentially curative resection, adjuvant therapy benefits stage II and III rectal cancers (3). Adjuvant radiotherapy alone decreases local recurrences without survival benefit (4). Only chemotherapy combined with radiotherapy has consistently demonstrated the efficacy in the incidence of pelvic recurrence, disease free survival, and overall survival (5). However, it is still required to define the optimal combination of chemotherapy and radiotherapy, such as the drug regimen, the route of delivery, and the sequence of chemotherapy and radiotherapy. The intravenous infusion of 5-FU is known to be effective after concurrent chemoradiation. However, chemotherapy has often been limited by its toxicity and poor patient compliance. Doxifluridine (5'-DFUR; Furtulon™) is a synthetic 5-deoxynucleoside derivative. In animal models, doxifluridine has been shown to be present at higher concentrations in tumor tissues resulting in the higher FU concentration and cytotoxic effects in tumors (6). Recently, oral doxifluridine has been reported to have several advantages over conventional intravenous 5-FU: oral doxifluridine has lower toxicity and better quality of life without compromising the efficacy of the treatment (6).
The spell out phase (TNM) system is the widely accepted standard for staging cancer. It has long been recognized that the stage III rectal cancer patients are a heterogeneous group. Although adjuvant therapy has been shown to be beneficial for TNM stage III colorectal cancer, the five-year survival in such patients varies widely. Such wide variation in the survival may be due to the lack of the stratification of the depth of invasion and the degree of lymph node involvement (7). The substaging systems therefore, are required to compare treatment results and to predict outcome accurately. In the new AJCC staging system, the status of lymph node metastasis was included as follows. N1: metastasis in one to three lymph nodes. N2: metastasis in four or more nodes. Stage IIIA was defined T1-T2 N1 M0, stage IIIB was T3~T4 N1 M0, and stage IIIC was any T N2 M0 (8).
In this study, we explored treatment outcome by the new AJCC staging system and compared treatment outcome of oral doxifluridine and the standard Mayo Clinic regimen as adjuvant chemotherapy after chemoradiation in resectable rectal cancer.
All the patients in this study had histologically confirmed adenocarcinoma of the rectum. They had undergone curative resection and without gross microscopical evidence of residual disease. Patients diagnosed as the stage II and III were enrolled. The eligibility criteria were adequate performance status (Karnofsky score 80 or higher) and normal hepatic, renal and bone marrow function. The exclusion criteria were previous radiation to the pelvis, previous chemotherapy, other malignant disease within the previous five years, distant metastasis, or severe coexistent disease. Written informed consent was obtained from all patients prior to the study entry.
The combination treatment of postoperative local radiation and systemic therapy with a 5-FU based regimen. Three weeks after operation, patients were treated with 5-FU 375 mg/m2/day intravenously for the first 3 days and the last 3 days of radiotherapy. Radiotherapy was given with a linear accelerator. Patients were treated with a three-field plan (posteroanterior and two lateral wedge field). The top of the field was placed at the L5/S1 junction, the lateral border was placed at 1.5 cm lateral to the bony pelvis, the inferior margin was placed at the inferior margin of the obturator foramen or 3 cm below the lowest tumor margin, the anterior border was placed at 3 cm anterior to the tumor mass, and the posterior border was placed at 0.5 cm posterior to the sacrum surface. Radiation therapy was administered 5 days a week for 5 weeks with 1.8 Gy fractionation daily. The total dose in the entire pelvis was 45 Gy. The boost dose 5.4 Gy in three fractions were given to reduced field that encompassed the tumor bed and adjacent lymph nodes with the margin 2 cm. After the completion of chemoradiation, patients were treated with 6 cycles of the Mayo Clinic regimen (5-FU 425 mg/m2/day and leucovorin 20 mg/m2/day intravenous bolus infusion for 5 days every 4 weeks) or oral doxifluridine (600 mg/m2/day) for 12 months.
Side effects were graded according to the National Cancer Institute-Common Toxicity Criteria (NCI-CTC) in all patients. If f the grade III leukopenia or diarrhea occurred, chemotherapy was discontinued until the recovery to the grade I or better and the treatment was resumed with the dose reduced by 20%.
During adjuvant therapy, patients were monitored for signs of the toxicity of their chemotherapy and radiotherapy with appropriate adjustments. Complete blood counts were performed weekly to detect the myelosuppression. In addition, prior to each cycle of chemotherapy, patients were evaluated by medical history, physical examination, complete blood counts, and blood chemistry studies. After the completion of adjuvant treatment, we regularly evaluated patients for disease recurrence. Every 3 months for the first 24 months after surgery and subsequently every 6 months for 5 years, medical history taking, physical examination, complete blood counts, blood chemistry studies, carcinoembryonic antigen (CEA), and chest radiography were performed. Computed tomography of the abdomen and pelvis was performed every 6 months for the first 2 years after surgery and subsequently every 12 months. Colonofiberoscopy or radiography of the colon was performed every 12 months.
Disease-free survival was defined as the duration from surgery to initial disease recurrence. Overall survival was defined as the duration from surgery to death. The Kaplan-Meier method was used to construct the curve for the disease free survival and overall survival. The data of patients who died without the evidence of disease recurrence were censored at the time of death in the calculations of disease-free survival. The log-rank test was used to compare distributions. The Cox proportional hazards model was used for all multivariate analyses. All data were analyzed using SPSS software (version 10.0).
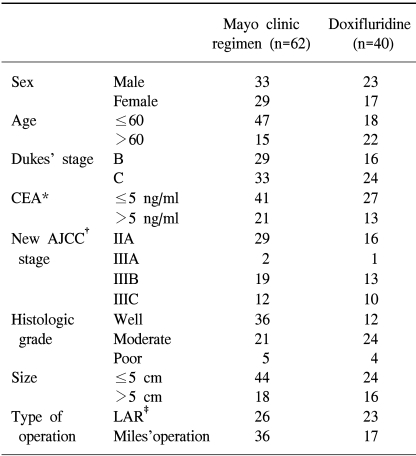
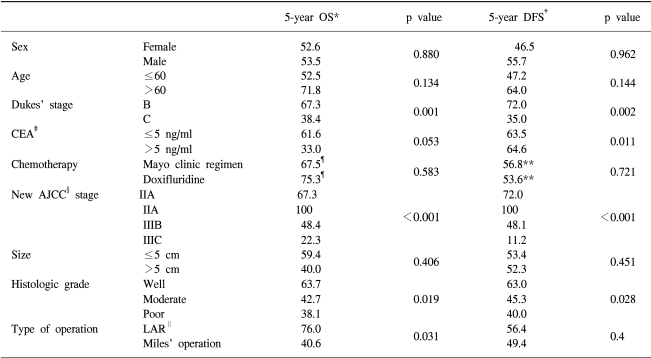
Between January 1995 and January 2002, a total of 109 patients were enrolled in the study. 7 patients (6.4%) were ineligible for the following reasons: 5 patients refused to take their assigned treatment and two patients died due to postoperative complications. 102 patients thus were eligible for follow-up and included in the statistical analysis. The median age of the patients was 56 years, ranged from 29~76 years. Approximately 60.8% patients received Mayo Clinic adjuvant chemotherapy, the rest received oral doxifluridine medication. The main characteristic of the patients in both treatment groups was comparable except histologic grade (p=0.019) and age (p=0.002) (Table 1).
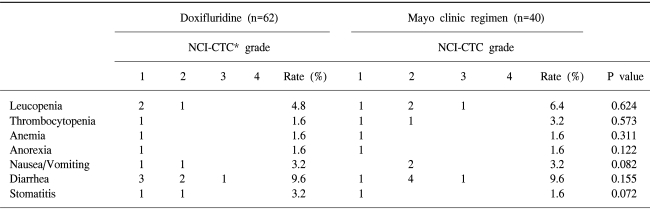
The adverse drug reactions were shown in Table 2. The grade III or IV toxicity was detected in 1.61% patients (1/62) in the doxifluridine group and 5% patients (2/40) in the Mayo Clinic group. The difference in the toxicity was not significant between two groups (p=0.541). The most frequent adverse drug reaction was gastrointestinal disturbance and bone marrow suppression in both groups. Gastrointestinal and mucocutaneous toxicy was mild in most cases. The major hematologic side effect was leukopenia. However, death related to the treatments did not occur.
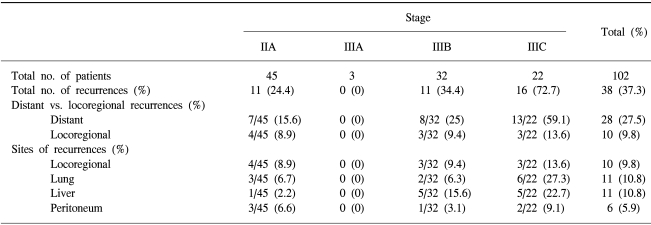
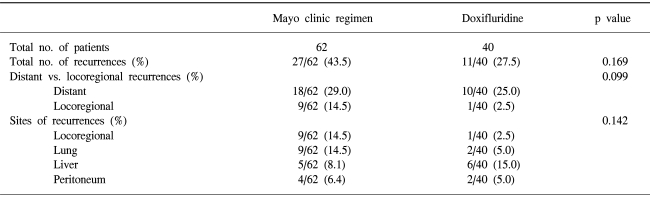
The median follow-up duration was 30 months. The disease recurred in 38 patients (37.3%). The relapse pattern is shown in Table 3 and Table 4. The locoregional recurrence rate was 8.9% (4/45) in the stage IIA, 0% (0/3) in the stage IIIA, 9.4% (3/32) in the stage IIIB and 13.6% (3/22) in the stage IIIC (p=0.148). The systemic failure was 15.6% (7/45) in the stage IIA, 0% (0/3) in the stage IIIA, 25% (8/32) in the stage IIIB, and 59.1% (13/22) in the stage IIIC (p=0.048)(Table 3). The relapse rate in the Mayo clinic regimen group was 43.5%, and the doxifluridine group was 27.5%. The difference in the two groups was not significant (p=0.169). The distant metastasis rate was also comparable in the two groups. The locoregional failure rate in the Mayo clinic regimen group was higher than the doxifluridine group (14.5% vs. 2.5%). The difference, however, was not statistically insignificant (p=0.099) (Table 4).
The liver and lung were the most common sites of the first relapse. 19 relapsed patients were treated with oxaliplatin plus 5-FU and leucovorin (oxaliplaitn group). 12 relapsed patients were treated with other regimens (other regimen group), irinotecan-based chemotherapy or hepatic arterial infusion of FUDR. 7 patients were not treated. The median overall survival was 28.9 months in the oxaliplatin group and 18.2 months in the other regimen group. The difference of the median survival in the two groups was not statistically significant (p=0.834). The median disease free survival was 12 months in the oxaliplatin group and 12.7 months in other regimen group. The difference was not statistically significant (p=0.837). The data demonstrate that the treatment modality did not influence the treatment outcome in the relapsed patients.
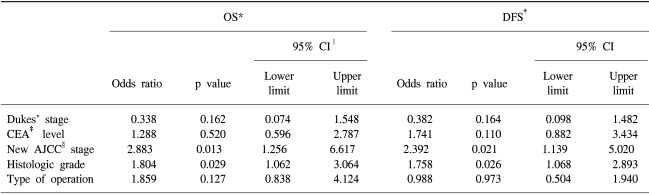
5 year disease-free survival was 52.0% and 5 year overall survival was 56.0%. Prognostic variables such as sex, age, preoperative CEA level, tumor size, histologic grade, Dukes' stage, new AJCC staging, type of surgical resection, and chemotherapy were entered into the univariate analysis (Table 5). The 5-year disease-free survival (DFS) rate was significantly higher in the stage IIA/IIIA than stage IIIB/IIIC (72%/100% vs 48.1%/11.2%, p<0.001). The 5-year overall survival (OS) rate was also significantly higher in the IIA/IIIA stage than the IIIB/IIIC stage (67.3%/100% vs 48.4%/22.3%). The difference was significant (p<0.001) (Fig. 1). However, the difference in 3-year DFS and OS between the oral doxifluridine group and the Mayo Clinic regimen group was not significant (p=0.721 and p=0.583, respectively) (Fig. 2). When we analyzed other prognostic factors for overall survival, Dukes' stage, histologic grade and type of surgical resection were moderately significant. In DFS, CEA level and histologic grade were moderately significant (Table 5). When these variables were entered into the multivariate analysis, the new AJCC staging and histoloic grade were independent prognostic factors in the overall survival and the disease free survival (p=0.013 and p=0.029, respectively) (Table 6).
The combined-modality therapy is the standard adjuvant treatment for rectal cancer patients with T3/4 or N+ disease (9). Postoperative radiation appears to be a survival advantage when it is combined with chemotherapy in high risk rectal cancer. The efficacy of the combined modality of the 5-FU-based regimen was established in a series of prospective randomized trials (5,10). Radiation therapy decreases local recurrence. Combined with chemotherapy, radiation which further decreases local recurrence to approximately 10%. This is responsible for the increase of the overall 5-year survival approximately 10~15% above the 5-year survival of surgery alone (3,5,10). Many chemotherapy regimens have been examined in the adjuvant therapy of rectal cancer, although virtually all regimens have been based on 5-FU (11). From the mid-1990s, the combination of 5-FU and leucovorin was introduced worldwide as the standard postoperative adjuvant therapy for colorectal cancer (12). 5-FU has been used in a variety of schedules. The continuous infusion of 5-FU has led to a more favorable toxicity profile and higher response rate than bolus 5-FU (13). However, the continuous infusion of 5-FU is not routinely administered because of its high cost, marginal survival benefit, and catheter-associated complications such as thrombosis, infection, and bleeding that may have a negative impact on quality of life. The use of oral chemotherapy provides convenience and cost savings by eliminating the necessity of hospital visit for intravenous chemotherapy. The patient's preference has been shown to be very strongly in favor of the oral agent, 89% patients preferred oral chemotherapy (14). Doxifluridine is a fluoropyrimidine derivative. The systemic availability of oral doxifluridine ranges from 50% to 80% with the therapeutic index 10 to 15 times higher than 5-FU in animal models (15). Doxifluridine is converted to 5-FU by thymidine phosphorylase. The activity thymidine phosphorylase in tumor tissues is higher than normal tissues resulting in higher anti-tumor effects in tumor tissues (16). One of the advantages of oral doxifluridine is that it can provide the prolonged 5-FU exposure at the lower peak concentration than bolus intravenous administration, which mimics the pharmacokinetics of continuous infusion of 5-FU. When intravenous 5-FU and oral doxifluridine were compared as a preoperative concurrent chemoradiation for locally advanced rectal cancer, treatment outcome and toxicity were comparable in these two regimens (17).
The optimal dose or administration schedule for oral doxifluridine was not established. However, previous studies indicated that the regimen 800 mg/m2/day daily and the regimen between 1,000 and 1,400 mg/m2/day in the intermittent schedule had antitumoral activity with acceptable side effects (6). In this study, relatively low dose (600 mg/m2/day) of doxifluridine was given because of previous chemoradiaton treatment. However, treatment results were comparable to previous results (11). The antitumor activity of doxifluridine and its low myelotoxicity has been attributed to cell metabolism. In some experiments, after administration of doxifluridine, the concentration of 5-FU in tumor cells was higher than administration of 5-FU itself (18).
In this study, in patients treated with the Mayo clinic regimen and doxifluridine, age and histologic grade were different because the data were reviewed retrospectively. Patients treated with doxifluridine had tumors with higher histologic grade and older than the Mayo clinic regimen group. However the difference of relapse rate was not different: DFS (p=0.721), OS (p=0.583). In addition, side effects between two groups were not different. The major gastrointestinal disturbances were nausea, vomiting and diarrhea. The major hematologic side effect was leukopenia. The toxicitywas generally mild and reversible in both treatment groups.
The previous TNM staging of colon cancer have grouped patients having mesenteric nodal involvement into stage III category despite variations in the depth of tumor penetration of the colonic wall (19). And medium-sized patient data has suggested that stage III colon cancer is heterogenous in survival when the difference in T and N categories are considered (7). Recently 50,042 stage III colon cancer patients using the new TNM staging system were evaluated (20). In this trial, survival rate was calculated by dividing stage III patients into 3 new subgroups (IIIA: T1-2 N1; IIIB: T3-4 N2; IIIC: Any T N2). They showed that 5-year survival rate of these subgroups were different. 59.8% in the IIIA, 42.0% in the IIIB and 27.3% in the IIIC (p<0.0001). They also evaluated 5,988 stage III rectal cancer (21). These 3 subgroups differed in the five-year survival rate. The IIIA was 55.1%, the IIIB was 35.3% and the IIIC was 24.5% (p<0.0001). This is in agreement with the previous result of colon cancer study (20). These results support the stratification of stage III patients into 3 subgroup (A, B, and C), which is now the new staging system (8). They also suggested that future adjuvant therapy trials in rectal cancer should incorporate the new staging system and, particularly, stage IIIC patients should be selected for aggressive therapy as they have an extremely poor prognosis. Our study showed the similar results although the number of stage IIIA patients was small. In our study, Dukes' stage was not a significant factor in the multivariate analyses despite its great significance in the univariate analysis. Therefore, the new AJCC staging system was a superior prognostic factor in rectal cancer patients.
The new AJCC staging system is superior to Dukes' system in prognostic stratification. Adjuvant therapy trials in rectal cancer should incorporate the new AJCC staging system, particularly stage IIIC patients should be selected for aggressive therapy as they have an extremely poor prognosis.
Our data showed that oral doxifluridine is comparable to the Mayo Clinic regimen in terms of DFS and OS as adjuvant chemotherapy of the rectal cancer when combined with postoperative chemoradiation.
Acknowledgement
This work was supported by a grant from the Korea Science & Engineering Foundation (KOSEF) through the MRC Center at Dong-A University.
References
1. Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001; 51:15–36. PMID: 11577478.

2. Bae JM, Won YJ, Jung KW, Park JG. Annual report of the Korea central cancer registry program 2000: based data from 131 hospitals. Cancer Res Treat. 2002; 34:77–83.
3. Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW, Kubista TP, Poon MA, Meyers WC, Mailliard JA. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991; 324:709–715. PMID: 1997835.

4. Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: A systematic overview of 8,507 patients from 22 randomised trials. Lancet. 2001; 358:1291–1304. PMID: 11684209.
5. O'Connell MJ, Martenson JA, Wieand HS, Krook JE, Macdonald JS, Haller DG, Mayer RJ, Gunderson LL, Rich TA. Improving adjuvant therapy for rectal cancer by combining protracted-infusion 5-FU with radiation therapy after curative surgery. N Engl J Med. 1994; 331:502–507. PMID: 8041415.
6. Neri B, Gemelli MT, Pantalone D, Pernice ML, Agostino I, Scatizzi M, Siliani GM, Bartolini P, Andreoli F. Results of leucovorin and doxifluridine oral regimen in the treatment of metastatic colorectal caner. Anticancer Drugs. 1998; 9:599–602. PMID: 9773803.
7. Merkel S, Mansmann U, Papadopoulos T, Wittekind C, Hohenberger W, Hermanek P. The prognostic inhomogeneity of colorectal carcinomas Stage III: a proposal for subdivision of Stage III. Cancer. 2001; 92:2754–2759. PMID: 11753948.
8. American Joint Committee on Cancer. AJCC cancer staging manual. 2002. 6th ed. New York: Springer-Verlag;p. 113–123.
9. National Institutes of Health Consensus Conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990; 264:1444–1450. PMID: 2202842.
10. Fisher B, Wolmark N, Rockette H, Redmond C, Deutsch M, Wickerham DL, Fisher ER, Caplan R, Jones J, Lerner H. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst. 1988; 80:21–29. PMID: 3276900.
11. Tepper JE, O'Connell M, Niedzwiecki D, Hollis DR, Benson AB III, Cummings B, Gunderson LL, Macdonald JS, Martenson JA, Mayer RJ. Adjuvant therapy in rectal cancer: Analysis of stage, sex, and local control?final report of intergroup 0114. J Clin Oncol. 2002; 20:1744–1750. PMID: 11919230.

12. International multicentre pooled analysis of colon cancer trials (IMPACT) investigators. Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. Lancet. 1995; 345:939–944. PMID: 7715291.
13. Meta-analysis Group in Cancer. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol. 1998; 16:301–308. PMID: 9440757.
14. Liu G, Franssen E, Fitch MI, Warner E. Patient preference for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997; 15:110–115. PMID: 8996131.
15. Bollag W, Hartmann HR. Tumor inhibitory effects of a new fluorouracil derivative: 5'-deoxy-5-fluorouridine. Eur J Cancer. 1980; 16:427–432. PMID: 6447071.

16. Kono A, Hara Y, Suga S, Karube Y, Matsushima Y, Ishitsuka H. Activation of 5'-deoxy-5-fluorouridine by thymidine phosphorylase in human tumors. Chem Pharm Bull. 1983; 31:175–178. PMID: 6221809.

17. Kim NK, Min JS, Park JK, Yun SH, Sung JS, Jung HC, Roh JK. Intravenous 5-FU versus oral doxifluridine as preoperative concurrent chemoradiation for locally advanced rectal cancer: Prospective randomized trials. Jpn J Clin Oncol. 2001; 31:25–29. PMID: 11256837.
18. Armstroung RD, Cadman E. 5'-Deoxy-5-fluorouridine selective toxicity for human tumor cells compared to human bone marrow. Cancer Res. 1983; 43:2525–2528. PMID: 6221792.
19. Tang R, Wang JY, Chen JS, Chang-Chien CR, Tang S, Lin SE, You YT, Hsu KC, Ho YS, Fan HA. Survival impact of lymph node metastasis in TNM stage III carcinoma of the colon and rectum. J Am Coll Surg. 1995; 180:705–712. PMID: 7773484.
20. Greene FL, Stewart AK, Norton HJ. A new TNM staging strategy for node-positive (stage III) colon cancer: An analysis of 50,042 patients. Ann Surg. 2002; 236:416–421. PMID: 12368669.
21. Greene FL, Stewart AK, Norton HJ. A new TNM staging strategy for node-positive (stage III) rectal cancer: An analysis of 5,988 patients. Proc Am Soc Clin Oncol. 2003; 22:251.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download