Abstract
Background:
Follicular lymphomas present with various immunohistologic patterns. The immunohistochemical markers used in the diagnosis of follicular lymphoma show variable degrees of sensitivity and specificity, and thus, additional germinal center markers are required. Smad1 has been reported to be overexpressed in follicular lymphoma, but little is known regarding the expression patterns of Smad proteins in human lymphoid tissue.
Methods:
In the present study, we performed immunohistochemistry for traditional germinal center markers and for Smad1 in human reactive lymphoid and follicular lymphoma tissues to investigate Smad1’s usefulness in the diagnosis of follicular lymphoma.
Results:
In the reactive germinal centers, most cells were positive for Smad1. Among the 27 follicular lymphoma cases, 17 of 21 (80%) were Smad1 positive, 17 of 27 (63%) were positive for CD10, and 23 of 27 (85%) were positive for Bcl6. Notably, three cases expressed CD10 only, and one only expressed Bcl6. All these cases were grade 3 tumors and showed follicular and diffuse growth patterns.
Follicular lymphoma (FL) is the neoplastic counterpart of normal germinal center (GC) B-cells and can present with various immunohistologic patterns which make them difficult to distinguish from other B-cell lymphomas [1]. Of the GC markers used in the diagnosis of FL, CD10 is a traditional marker of GC B cells and has long been regarded as a reliable marker of both GCs and FL [2]. The reported CD10 positivity rates in FL vary widely, though most reports indicate positivity rate of 60% to 90% [3]. Furthermore, CD10 showed less expression in grade 3 FL than in indolent grades 1 or 2 FL [4].
Another marker for GC B cells is the Bcl6 protein. The intense and diffuse staining for this protein can also identify tumors arising from follicular GCs [5]. Bcl6 expression is most prominent in FL and displays an expression pattern similar to that of GCs [2]. Immunohistochemical staining for Bcl6 and CD10 can identify tumors of GC B-cell origin, and their co-expression has generally been used as a marker of tumors with a GC B-cell origin [2].
The immunohistochemical markers typically used in the diagnosis of FL, such as CD10 and Bcl6, show variable degrees of sensitivity and specificity and lack concordance of expression [1]. Thus, additional GC markers are needed. Recently, several other markers such as human GC associated lymphoma (HGAL), LIM-only transcriptional factor 2 (LMO2), and interferon regulatory factor 8 (IRF 8), have been explored [1]. In the search for new GC markers, Smad1 was found to be overexpressed in FL compared to normal GC B cells in a gene expression profiling study, suggesting its possible utility [6]. In the present study, we performed immunohistochemistry for Smad1 in human FL tissues to investigate its usefulness for the diagnosis of FL, and we compared these findings to other traditional GC markers.
We retrieved paraffin-embedded tissue blocks of 27 FL cases from the surgical files at our hospital. All cases were diagnosed according to the World Health Organization (WHO) criteria [7]. The B-cell nature of these tumors was confirmed by the immunohistochemical detection of B-cell (CD20) and T-cell (CD3) markers in the paraffin-embedded sections. Tumors were graded as grade 1, 2, or 3 according to the proportion of large cells (centroblasts). In addition, tumors were classified as follicular, follicular and diffuse, or focally follicular according to the proportion of follicular pattern present. To distinguish large, confluent follicles or interfollicular involvement from diffuse areas, we stained for follicular dendritic cells using CD21. The study subjects consisted of 17 male and 10 female patients with ages ranging from 23 to 68 years (mean, 47 years). Twenty-one tumors were located in lymph nodes and six were in extranodal sites (tonsil, 5; maxilla, 1). Reactive lymph node tissues were used as a control.
Immunohistochemical analysis was performed using formalin-fixed, paraffin-embedded materials with primary antibodies to CD10 (1:100, Novocastra Laboratories Ltd., Newcastle upon Tyne, UK), Bcl6 (1:600, Novocastra Laboratories Ltd.), and Smad1 (1:400, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). Briefly, sections were deparaffinized in xylene, rehydrated, washed in distilled water, immersed in 10 mM citrate buffer (pH 6), and either microwaved or autoclaved. They were then treated with 3% hydrogen peroxide solution to reduce endogenous peroxidase activity, washed in phosphate-buffered saline, and incubated with the primary antibodies. Bound antibodies were detected by the streptavidin biotin method using an LSAB kit (Dako Co., Carpinteria, CA, USA). The sections were then treated with diaminobenzidine and counterstained with Mayer hematoxylin. Tumor cells were considered positive only when we observed distinct membranous or cytoplasmic staining for CD10 and Smad1 or distinct nuclear staining for Bcl6. Marker expression was considered positive when more than 20% of the neoplastic lymphocytes were stained.
The morphologic findings of the 27 FL cases are summarized in Table 1. There are seventeen grade 3 tumors, nine tumors with a follicular and diffuse growth pattern, and one tumor with a focally follicular growth pattern.
In reactive GCs, most cells (centrocytes and centroblasts) were positive for Smad1. No other cells expressed Smad1, with the exception of scattered plasma cells, which were more strongly positive than the GC cells (Fig. 1). Smad1 immunostaining was not performed in six cases of FLs because of a shortage of Smad1 antibody. Of the tested FL cases, 17 of 21 (80%) were Smad1+ (Fig. 2A). When we tested common GC markers, 17 of 27 (63%) were positive for CD10 (Fig. 2B) and 23 of 27 (85%) were positive for Bcl6 (Table 2, Fig. 2C). Of the 21 cases immunostained for all three antibodies, all of the grade 1 and 2 tumors were positive for Smad1, CD10, and Bcl6. For the 15 grade 3 tumors, three were positive for all three markers, eight co-expressed Smad1 and Bcl6, three expressed CD10 only and one expressed Bcl6 only (Table 3). Most tumors with a follicular growth pattern were positive for Smad1, CD10, and Bcl6 (9 of 11, 82%), and the remaining were positive for Smad1 and Bcl6 (n=2). However, of the nine tumors with a follicular and diffuse pattern, five co-expressed Smad1 and Bcl6, three expressed CD10 only, and one expressed Bcl6 only. The one case with a focally follicular pattern was positive for Smad1 and Bcl6 but was negative for CD10 (Table 4).
This immunohistochemical study was undertaken to investigate the usefulness of Smad1 for the diagnosis of FL in human reactive lymphoid and FL tissues and to compare Smad1 to the traditional GC markers. Our results indicate the Smad signaling pathway is involved in the maintenance of homeostasis in human lymphoid follicles, and our results demonstrate that Smad1 is a candidate GC marker. In addition, they suggest that the Smad signaling pathway might be functionally active in FL.
CD10 is a cell surface metalloproteinase that reduces the cellular responses to peptide hormones and is found on neutrophils, B lymphoblasts, some T lymphoblasts, normal follicular center cells, follicular helper T cells, and some nonhematopoietic cells [1,3]. CD10 is a traditional marker of GC B cells and has long been regarded as a reliable marker for both GC and FL [2]. Furthermore, its expression is well correlated with the t(14;18) (q32;q21) chromosomal translocation, which is the most reliable diagnostic criterion for FL [8]. DNA microarray analysis has also confirmed the association between CD10 and other genes associated with GC cells [9]. However, CD10 positivity in FL varies widely, and most reports on the topic indicate that 60% to 90% of tumors are CD10 positive [3]. Furthermore, CD10 expression is frequently weak to negative in WHO grade 3 FLs [10], and in one study, CD10 was detected in only 20% of these tumors [1]. Therefore, the lack of CD10 expression does not preclude FL [1] or exclude the possibility that neoplastic lymphocytes originated from follicular center cells in diffuse large B-cell lymphoma (DLBCL) [10]. In the present study, CD10 was positive in 63% of the 27 FL cases as follows: grade 1 (6 of 6, 100%), grade 2 (4 of 4, 100%), and grade 3 (7 of 17, 41%); follicular (14 of 17, 82%), follicular and diffuse (3 of 9, 33%), and focally follicular (0 of 1, 0%). Our findings are consistent with other studies [1] and confirm that CD10 expression is frequently weak to absent in high grade FL or in FL with a diffuse growth pattern.
Bcl6 protein is another GC B-cell marker [10], and its expression, which is independent of Bcl6 gene rearrangement, is largely restricted to GC B cells (centroblasts and centrocytes) in normal human lymphoid tissues [1,5]. Intense and diffuse staining for this protein can also identify tumors arising from follicular GCs [5]. In B-cell lymphoma, Bcl6 expression is most prominent in FL and Burkitt’s lymphoma, in which it displays a pattern of expression similar to that of GCs. Bcl6 staining has been useful in detecting tumors of GC B-cell derivation in DLBCL [2]. In addition, staining for Bcl6 and CD10 in combination can identify tumors of GC B-cell origin in archival specimens, and their co-expression is generally used as a tumor marker of tumor of GC B-cell origin [2]. Furthermore, Bcl6’s expression has been reported in CD10 negative, MUM1-positive FL, which frequently presents as high grade FL in the absence of the t(14:18) translocation. Bcl6 is a useful adjunct for the diagnosis of CD10 and/or Bcl2-negative FL [1] and is a more reliable marker than CD10, as it is conserved in high grade, interfollicular, and diffuse areas [11]. In the present study, Bcl6 was expressed in 85% of 27 FL cases as follows: grade 1 (5 of 6, 83%), grade 2 (4 of 4, 100%), and grade 3 (14 of 17, 82%); follicular (16 of 17, 94%), follicular and diffuse (6 of 9, 67%), and focally follicular (1 of 1, 100%). These results suggest that Bcl6 is superior to CD10, especially for the diagnosis of high grade FL or FL without a predominant follicular pattern.
Smad proteins play a key role in signal transduction of transforming growth factor beta (TGF-β) family members, including TGF-β and bone morphogenetic proteins [12,13]. Lymphoid tissues and stromal cells (in a mouse model), and T-cells of the developing thymus show widespread expression of the common mediator, Smad4, and show moderate expression of the TGF-β–specific Smads 2 and 3 [14]. Using gene expression profiling, Smad1 was found to be the most differentially overexpressed gene in FL compared to normal GC B cells [6]. However, little is known about the expression pattern of Smad proteins in human lymphoid tissue. In the present study, most GC cells (centrocytes and centroblasts) were positive for Smad1 in reactive GCs, and although scattered plasma cells were more strongly positive than GC cells, no other cells expressed Smad1. Furthermore, Smad1 expression was observed in 17 of 21 cases (80%) of FL, which is similar to the positive expression rate of Bcl6. Smad1 was positive in all grade 1 and 2 tumors and in 11 of 15 grade 3 tumors (73%). Regarding growth patterns, all tumors with a follicular pattern (11 of 11, 100%) and five of nine tumors with a follicular and diffuse pattern (56%) were Smad1-positive. One tumor showing a focally follicular growth pattern was also positive for Smad1. Therefore, the addition of Smad1 to the routine diagnostic panel for FL might be useful, especially for the diagnosis of high-grade tumors without a predominant follicular growth pattern. These findings can be extended to the identification of follicular origin tumors in DLBCL. Further studies are needed to determine that Smad1 expression shows higher sensitivity and specificity in FL than those in other B-cell lymphomas, including mantle cell lymphoma, MALT lymphoma, small lymphocytic lymphoma.
TGF-β is a potent growth inhibitor of most cells, and cellular insensitivity to growth inhibition by TGF-β is a hallmark in the genesis and progression of human cancer. This can be directly linked to inactivating mutations or the loss of expression of various signaling molecules. Therefore, TGF-β and its signaling proteins are regarded as widely established tumor suppressors [15]. The major effects of TGF-β in B-cells are the inhibition of DNA synthesis and the induction of cell cycle arrest, in addition to the induction of apoptosis in some B-cell lines [6]. FLs are the neoplastic counterparts of normal GC B cells [1], and the overexpression of Bcl-2 in FL cells due to t(14;18) [16] may prevent TGF-β–induced apoptosis [6].
Although extensively investigated in solid tumors, there have not been many reports of the Smad-associated pathways in B-cell lymphoma [17]. Using gene expression profiling, Smad1 was found to be the most differentially overexpressed gene in FL compared to the normal GC B cells [6]. The overexpression of Smad1 may increase the sensitivity of FL cells to the effects of TGF-β and contribute to the hypoproliferative nature of these cells [6]. One study reported that 13 of 29 (45%) FL or transformed FL samples showed weak to moderate intensity nuclear staining in more than 10% of cells by immunostaining for phosphospecific Ser463/465 Smad1 (Smad1-P) antibody (Cell Signaling Technology, Beverly, MA, USA), which is not presently commercially available. Normal lymphoid tissue was wholly negative or rarely positive for GC cells. In that study, the presence of an active Smad1 pathway was suggested in FL but not in reactive B cells [16]. In the present study, Smad1 expression was observed in 17 of 21 FL cases (80%), using Smad1 (A-4) antibody (sc-7965), which detects cytoplasmic Smad1. Furthermore, many Smad1-positive tumors (10 of 21, 48%) showed strong or moderate positivity in the majority of the tumor cells.
TGF-β signaling through a family of transmembrane receptors and ligand binding to type II receptors results in the recruitment and transphsphorylation of type I receptors that then signal downstream responses and induce phosphorylation of Smad proteins [15]. Once phosphorylated, R-Smads, such as Smad1, associate with Smad4, translocate to the nucleus, and regulate the TGF-β pathway [16]. However, a part of the phosphorylated Smad1 remains in the cytoplasm as well. Taken together, these results suggest that the Smad signaling pathway might be involved in this type of lymphoma. Investigation of the expression of other proteins in the Smad signaling pathway and molecular studies are warranted to determine the mechanisms of the Smad1-specific pathway in FL.
REFERENCES
1. Younes SF, Beck AH, Lossos IS, Levy R, Warnke RA, Natkunam Y. Immunoarchitectural patterns in follicular lymphoma: efficacy of HGAL and LMO2 in the detection of the interfollicular and diffuse components. Am J Surg Pathol. 2010; 34:1266–76.

2. Kwon MS, Go JH, Choi JS, et al. Critical evaluation of Bcl-6 protein expression in diffuse large B-cell lymphoma of the stomach and small intestine. Am J Surg Pathol. 2003; 27:790–8.

3. King BE, Chen C, Locker J, et al. Immunophenotypic and genotypic markers of follicular center cell neoplasia in diffuse large B-cell lymphomas. Mod Pathol. 2000; 13:1219–31.

4. Borovecki A, Korać P, Nola M, Ivanković D, Jaksić B, Dominis M. Prognostic significance of B-cell differentiation genes encoding proteins in diffuse large B-cell lymphoma and follicular lymphoma grade 3. Croat Med J. 2008; 49:625–35.

5. Ree HJ, Yang WI, Kim CW, et al. Coexpression of Bcl-6 and CD10 in diffuse large B-cell lymphomas: significance of Bcl-6 expression patterns in identifying germinal center B-cell lymphoma. Hum Pathol. 2001; 32:954–62.

6. Husson H, Carideo EG, Neuberg D, et al. Gene expression profiling of follicular lymphoma and normal germinal center B cells using cDNA arrays. Blood. 2002; 99:282–9.

7. Harris NL, Swerdlow SH, Jaffe ES, et al. Follicular lymphoma. Swerdlow SH, Campo E, Harris NL, editors. WHO classification of tumors of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC Press;2008. p. 220–6.
8. Straka C, Mielke B, Eichelmann A, Trede I, Ho AD, Möller P. Bcl-2 gene rearrangements in primary B-cell lymphoma of the gastrointestinal tract reveal follicular lymphoma as a subtype. Leukemia. 1993; 7:268–73.
9. Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000; 403:503–11.

10. Eshoa C, Perkins S, Kampalath B, Shidham V, Juckett M, Chang CC. Decreased CD10 expression in grade III and in interfollicular infiltrates of follicular lymphomas. Am J Clin Pathol. 2001; 115:862–7.

11. Goteri G, Lucarini G, Zizzi A, et al. Comparison of germinal center markers CD10, BCL6 and human germinal center-associated lymphoma (HGAL) in follicular lymphomas. Diagn Pathol. 2011; 6:97.

12. Yue J, Frey RS, Mulder KM. Cross-talk between the Smad1 and Ras/MEK signaling pathways for TGFbeta. Oncogene. 1999; 18:2033–7.
13. Kretzschmar M, Liu F, Hata A, Doody J, Massagué J. The TGF-beta family mediator Smad1 is phosphorylated directly and activated functionally by the BMP receptor kinase. Genes Dev. 1997; 11:984–95.

14. Flanders KC, Kim ES, Roberts AB. Immunohistochemical expression of Smads 1-6 in the 15-day gestation mouse embryo: signaling by BMPs and TGF-betas. Dev Dyn. 2001; 220:141–54.
15. Schiemann WP, Pfeifer WM, Levi E, Kadin ME, Lodish HF. A deletion in the gene for transforming growth factor beta type I receptor abolishes growth regulation by transforming growth factor beta in a cutaneous T-cell lymphoma. Blood. 1999; 94:2854–61.
16. Munoz O, Fend F, de Beaumont R, Husson H, Astier A, Freedman AS. TGFbeta-mediated activation of Smad1 in B-cell non-Hodgkin’s lymphoma and effect on cell proliferation. Leukemia. 2004; 18:2015–25.
17. Go JH. Expression pattern of Smad proteins in diffuse large B-cell lymphomas. Korean J Pathol. 2004; 38:301–5.
Fig. 1.
Immunohistochemical pattern of Smad1 in reactive germinal centers. Most centrocytes and centroblasts are positive for Smad1 with moderate intensity. Scattered plasma cells are more strongly positive than germinal center cells (A, B).
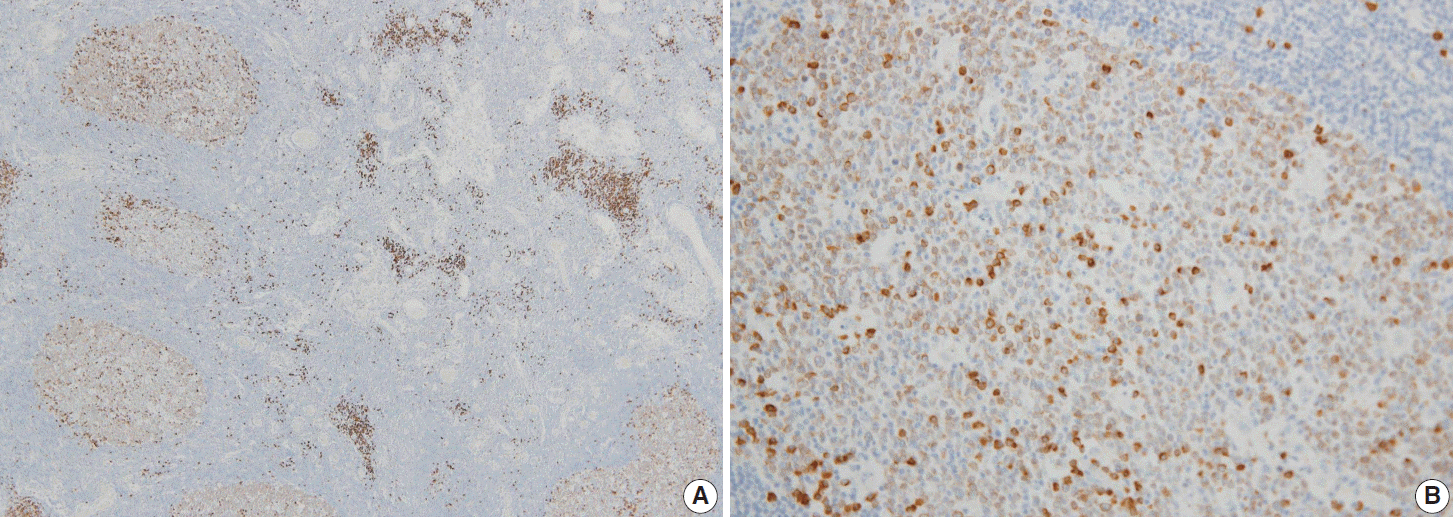
Fig. 2.
Immunohistochemical pattern of follicular lymphoma. The tumor cells are positive for Smad1 (A), CD10 (B), and Bcl6 (C).
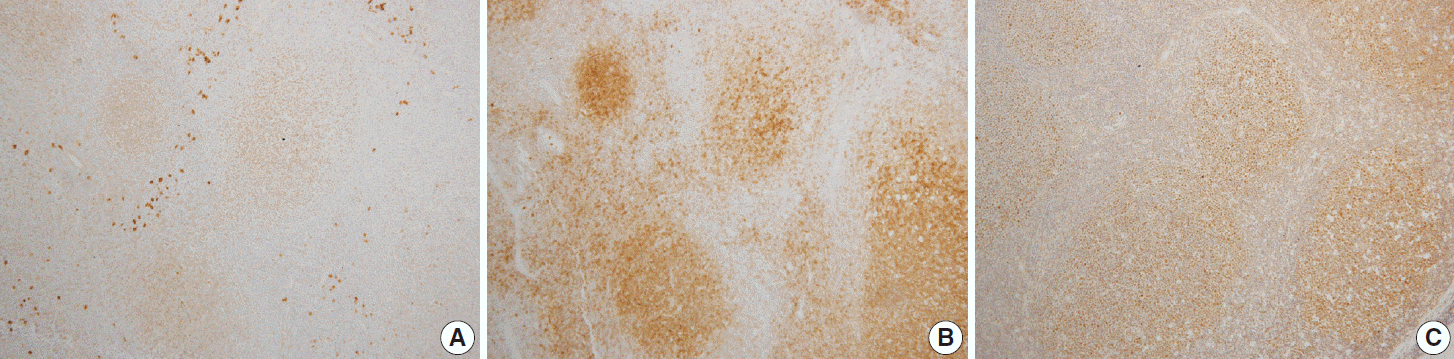
Table 1.
Morphologic and immunohistochemical findings for follicular lymphomas
Table 2.
Summary of Immunohistologic results for follicular lymphomas
| CD10 | Bcl6 | Smad1 | |
|---|---|---|---|
| Positive | 17 | 23 | 17 |
| Negative | 10 | 4 | 4 |
| Positive rate (%) | 63 | 85 | 80 |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download