Abstract
Oncocytic lipoadenoma is a rare tumor, with only 18 cases having been reported since the first in 1998. We encountered a case of oncocytic lipoadenoma presenting as a slowly growing parotid mass in a 71-year-old man. This tumor is characteristically comprised of a mixture of oncocytes and adipocytes. The present case is one of five reported cases of oncocytic lipoadenoma showing sebaceous differentiation. The results of immunohistochemical study with DOG1 antibody supported the origination of this tumor in the striated duct.
Oncocytic lipoadenoma is a rare entity, with only 18 cases having been reported in the literature. Oncocytic lipoadenoma is a benign encapsulated tumor composed of oncocytes and adipocytes, occurring mostly in parotid glands or less frequently in submandibular glands. We herein present a case of parotid oncocytic lipoadenoma with sebaceous differentiation and immunohistochemical study.
A 71-year-old man presented with a four-year history of a right infra-auricular mass. The mass measured 4×3 cm and was nontender and movable. Head and neck computed tomography revealed a mass with heterogenous density and a fatty component (Fig. 1A). Partial parotidectomy was performed, revealing a solitary well-defined mass measuring 4.2×3.5×2.5 cm with a thin capsule. The cut surface was tan with yellow areas (Fig. 1B).
Microscopically, the mass was encapsulated and surrounded by normal parotid tissue (Fig. 1C). The tumor comprised oncocytic cells and fat cells with mature adipose tissue occupying about 50% to 60% of the tumor. The oncocytic cells were arranged in an acinar or glandular pattern (Fig. 2A) and contained abundant eosinophilic cytoplasm. Some oncocytic cells were smaller in size, with darker cytoplasm and pyknotic nuclei. Scattered sebaceous glands were also present (Fig. 2B). No pleomorphism, mitosis, necrosis, or capsular invasion was observed.
Immunohistochemically, the oncocytic cells were strongly positive for AE1/AE3 (1:1,000, Dako, Glostrup, Denmark) and cytokeratin 7 (1:1,000, Dako). Peripheral cells were stained by p63 (1:1,000, Dako) and cytokeratin 5/6 (1:200, Dako). Epithelial membrane antigen (1:200, Dako) stained the luminal surface and sebaceous glands. The oncocytic cells were negative for smooth muscle actin (1:1,000, Dako) and calponin (1:1,000, Dako). Cytokeratin 14 (1:400, Biogenex, The Hague, The Netherlands) expression was restricted to sites of sebaceous differentiation. An apical-luminal pattern of DOG1 (1:200, Leica, Newcastle upon Tyne, UK) staining was observed in the normal serous acini, while the tumor cells were negative (Fig. 2C, D). Based on the microscopic findings, we rendered a diagnosis of oncocytic lipoadenoma with sebaceous differentiation. No recurrence was present after a 19-month follow-up.
Oncocytic lipoadenoma was first reported by Hirokawa et al. in 1998 [1]. This rare tumor was not mentioned as an entity in the 2005 World Health Organization (WHO) histologic classification of tumors of salivary glands. In the English literature, 18 cases have been reported (Table 1) [1-12]. There is no gender predilection, and the patient ages range from 7 to 89 years (median of 55.5 years). All reported cases demonstrated benign clinical courses after excision. The universal microscopic finding in the literature was an encapsulated tumor comprising a mixture of oncocytic cells and fat cells. So-called dark cells were also common. Uncommon findings as sclerotic and polycystic changes ascribed to chronic involution, squamous metaplasia, lymphoid stroma, and metaplastic bone formation have been reported [1,2]. The presence of mitochondria in those oncocytic cells was confirmed by both immunohistochemical and ultrastructural studies [7]. Phosphotungstic acid hematoxylin staining and antimitochondrial antibody were utilized to demonstrate the presence of mitochondria [3,6-8]. Ultrastructural studies also provided evidence that oncocytic lipoadenoma may be derived from striated ductal cells based on similar histological features [7]. DOG1 expression was found in both normal and neoplastic counterparts of intercalated ducts and acinar cells, whereas striated duct cells were negative [13]. The oncocytic cells in our case were negative for DOG1, which supports the contention that striated ductal cells were the origin of this tumor.
Differential diagnoses include sialolipoma, oncocytoma, oncocytosis, sclerosing polycystic adenosis, and oncocytic metaplasia. The latter two entities do not form a discrete tumor mass. Sialolipomas comprise ductal, acinar, basal, and myoepithelial cell components [14]. Oncocytomas are also encapsulated tumors with immunohistochemical findings identical to those of oncocytic lipoadenomas, but they lack adipose tissue as their component cells. In addition, 20% of patients with oncocytomas have a history of radiation exposure [15]. The presence of sclerotic and polycystic change can be reminiscent of sclerosing polycystic adenosis, but oncocytic lipoadenomas lack xanthomatous and apocrine change, which is commonly seen in sclerosing polycystic adenosis. In addition, the presence of acini in sclerosing polycystic adenosis can be aided by DOG1 antibody.
Sebaceous differentiation is not an uncommon finding in salivary glands and can be found in sebaceous lymphadenoma, sebaceous adenoma, pleomorphic adenoma, oncocytoma, sialoblastoma, and Warthin tumor [15,16]. The majority of the reported oncocytic lipoadenomas including our case exhibit sebaceous differentiation.
Lipoadenomas are similar to adenolipomas of the breast, thyroid, and skin in that they all demonstrate a histological mixture of epithelial components and adipose tissue. In contrast to adenolipoma, which is considered to be a hamartoma, oncocytic lipoadenoma is believed to be a true neoplasm. Ilie et al. [6] identified a t(12;14) translocation in their case. An altered HMGA2 gene rearrangement is more commonly seen in lipoma, pleomorphic adenoma, and leiomyoma [6]. The cytogenetic findings support oncocytic lipoadenoma as a distinct neoplasm.
REFERENCES
1. Hirokawa M, Shimizu M, Manabe T, Ito J, Ogawa S. Oncocytic lipoadenoma of the submandibular gland. Hum Pathol. 1998; 29:410–2.

2. Agaimy A, Ihrler S, Märkl B, et al. Lipomatous salivary gland tumors: a series of 31 cases spanning their morphologic spectrum with emphasis on sialolipoma and oncocytic lipoadenoma. Am J Surg Pathol. 2013; 37:128–37.
3. Aouad R, Matar N, Sader-Ghorra C, Haddad A, et al. Pathology quiz case 1. Oncocytic lipoadenoma of the parotid gland. Arch Otolaryngol Head Neck Surg. 2008; 134:446–8. 446, 8.
4. Chahwala Q, Siddaraju N, Singh N, Goneppanavar M, Basu D. Fine needle aspiration cytology of oncocytic lipoadenoma of the parotid gland: report of a rare case. Acta Cytol. 2009; 53:437–9.
5. Devadoss CW, Murugan P, Basu D, Jagdish S. Oncocytic lipoadenoma of the parotid gland: a rare case report. J Clin Diagn Res. 2012; 6:1076–8.
6. Ilie M, Hofman V, Pedeutour F, Attias R, Santini J, Hofman P. Oncocytic lipoadenoma of the parotid gland: immunohistochemical and cytogenetic analysis. Pathol Res Pract. 2010; 206:66–72.

8. Klieb HB, Perez-Ordoñez B. Oncocytic lipoadenoma of the parotid gland with sebaceous differentiation. Study of its keratin profile. Virchows Arch. 2006; 449:722–5.

9. McNeil ML, Bullock MJ, Trites JR, Hart RD, Taylor SM. Oncocytic lipoadenoma of the parotid gland with sebaceous differentiation in a 73-year-old male. J Otolaryngol Head Neck Surg. 2010; 39:E48–50.
10. Mitsimponas KT, Agaimy A, Schlittenbauer T, Nkenke E, Neukam FW. Oncocytic lipoadenoma of the parotid gland: a report of a new case and review of the literature. Int J Clin Exp Pathol. 2012; 5:1000–6.
11. Pusiol T, Franceschetti I, Scialpi M, Piscioli I. Oncocytic sialolipoma of the submandibular gland with sebaceous differentiation: a new pathological entity. Indian J Pathol Microbiol. 2009; 52:379–82.

12. Tokyol C, Dilek FH, Aktepe F, Ayçiçek A, Altuntaş A. Oncocytic lipoadenoma of the parotid gland: a case report with fine needle aspiration cytology findings. Kulak Burun Bogaz Ihtis Derg. 2010; 20:146–9.
13. Chenevert J, Duvvuri U, Chiosea S, et al. DOG1: a novel marker of salivary acinar and intercalated duct differentiation. Mod Pathol. 2012; 25:919–29.

14. Nagao T, Sugano I, Ishida Y, et al. Sialolipoma: a report of seven cases of a new variant of salivary gland lipoma. Histopathology. 2001; 38:30–6.

15. Brandwein MS, Huvos AG. Oncocytic tumors of major salivary glands: a study of 68 cases with follow-up of 44 patients. Am J Surg Pathol. 1991; 15:514–28.
16. Barnes L. Pathology and genetics of head and neck tumours: World Health Orginization of tumors. Lyon: IARC Press;2005. p. 209–281.
Fig. 1.
Oncocytic lipoadenoma. (A) Computed tomography reveals a heterogeneous tumor with a fat component (arrow). (B) Grossly, the cut surface is a mixture of tan and yellow components. (C) The scanning view shows an encapsulated tumor with biphasic components and surrounded by normal acini.
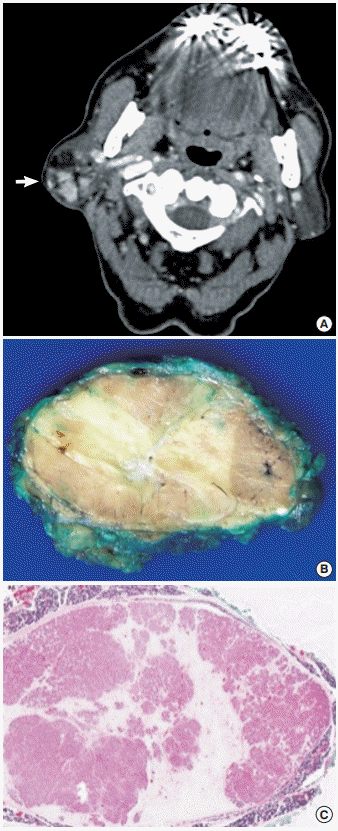
Fig. 2.
(A) Oncocytic cells are arranged in an acinar or glandular pattern. (B) The arrows indicate foci of sebaceous differentiation. DOG1 expression is seen in normal parotid acinar cells (C) but not oncocytic cells (D).
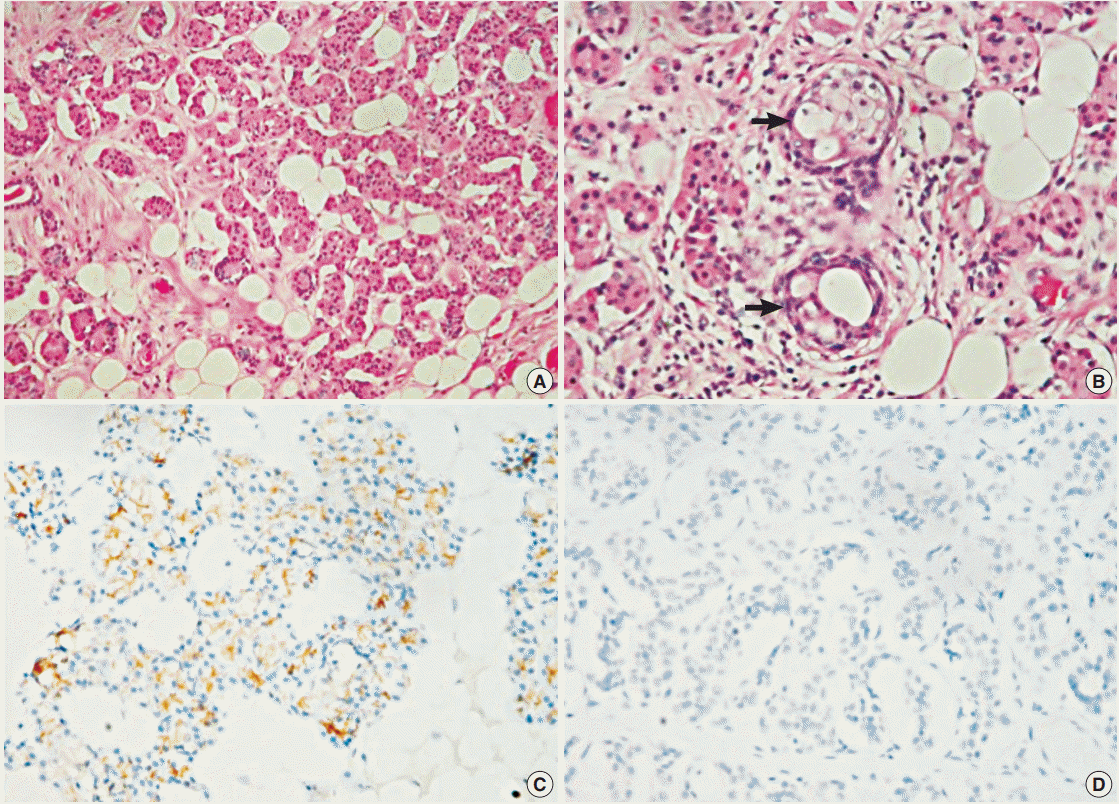
Table 1.
Clinical summary of the reported cases of oncocytic lipoadenoma
| Reference | Gender | Age (yr) | Location | Size (cm) | Sebaceous differentiation | Follow-up |
|---|---|---|---|---|---|---|
| Agaimy et al. [2] | M | 63 | Parotid gland | 4.5 | Present | NED, 6 mo |
| Agaimy et al. [2] | M | 29 | Parotid gland | 4.5 | Present | NED, 141 mo |
| Agaimy et al. [2] | F | 54 | Parotid gland | 2.9 | Absent | NED, 18 mo |
| Agaimy et al. [2] | F | 7 | Parotid gland | N/A | Present | N/A |
| Agaimy et al. [2] | F | 89 | Parotid gland | 4.2 | Present | N/A |
| Agaimy et al. [2] | M | 55 | Parotid gland | 2.7 | Present | NED, 13 mo |
| Aouad et al. [3] | M | 38 | Parotid gland | 4 | N/A | N/A |
| Chahwala et al. [4] | F | 50 | Parotid gland | 14 | N/A | N/A |
| Devadoss et al. [5] | F | 50 | Parotid gland | 13.5 | N/A | NED, 24 mo |
| Hirokawa et al. [1] | F | 66 | Submandibular gland | 11 | N/A | NED, 30 mo |
| Ilie et al. [6] | M | 64 | Parotid gland | 3.5 | Present | NED, 24 mo |
| Kato and Horie [7] | F | 57 | Parotid gland | 4.5 | N/A | N/A |
| Klieb and Perez-Ordonez [8] | F | 47 | Parotid gland | 3 | Present | NED, 6 mo |
| McNeil et al. [9] | M | 73 | Parotid gland | N/A | Present | N/A |
| Mitsimponas et al. [10] | F | 55 | Parotid gland | 2.7 | Present | NED, 12 mo |
| Pusiol et al. [11] | M | 73 | Submandibular gland | 9 | Present | N/A |
| Tokyol et al. [12] | M | 56 | Parotid gland | 7 | N/A | NED, 6 mo |
| Present case | M | 71 | Parotid gland | 4 | Present | NED, 19 mo |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download