This article has been
cited by other articles in ScienceCentral.
Abstract
Background:
Core needle biopsy is a relatively new technique used to diagnose salivary gland lesions, and its role in comparison with fine needle aspiration cytology needs to be refined.
Methods:
We compared the results of 228 ultrasound-guided core needle biopsy and 371 fine needle aspiration procedures performed on major salivary gland tumors with their postoperative histological diagnoses.
Results:
Core needle biopsy resulted in significantly higher sensitivity and more accurate tumor subtyping, especially for malignant tumors, than fine needle aspiration. No patient developed major complications after core needle biopsy.
Conclusions:
We recommend ultrasoundguided core needle biopsy as the primary diagnostic tool for the preoperative evaluation of patients with salivary gland lesions, especially when malignancy is suspected.
Go to :

Keywords: Salivary gland neoplasms, Biopsy, large-core needle, Biopsy, fine-needle, Parotid gland, Submandibular gland
In recent decades, fine needle aspiration cytology (FNAC) has been established as an efficient diagnostic tool for superficial masses, including salivary gland lesions. FNAC is technically simple, safe, fast, and cost-effective. However, FNAC traditionally demonstrates relatively low sensitivity in comparison with its high specificity for diagnosing salivary gland tumors. According to a previous meta-analysis by Schmidt
et al. [
1], the average sensitivity and specificity determined in 6,169 cases were 80% and 97%, respectively. Recent studies report that the sensitivity and specificity of FNAC range between 64%–90% and 86%–100%, respectively [
2-
8]. The low sensitivity of FNAC can be attributed to several factors, but is primarily due to the difficulty of diagnosing low-grade carcinomas by cellular morphology alone.
Core needle biopsy (CNB) is a relatively new technique for diagnosing salivary gland lesions. Since intact tissue cores can be retrieved using ultrasound-guided CNB, improved specimen adequacy is expected. The sensitivity and specificity of salivary gland CNB are reportedly 92%–94% and 99%–100%, respectively [
9-
12]. Preoperative evaluation of salivary gland lesions should provide the clinicians with a treatment plan including the type and extent of surgical intervention needed. For this purpose, differentiating benign from malignant tumors is crucial, and moreover, information on the grade and specific type of the tumor will further aid in the choice of therapeutic procedures.
With the aim of establishing the most accurate diagnostic tool as new techniques emerge, we compared the diagnostic accuracy and accurate tumor subtyping rates of CNB and FNAC performed for the preoperative evaluation of salivary gland tumors.
MATERIALS AND METHODS
Between July 2008 and June 2013, 708 tumors in the major salivary glands were surgically resected from 705 patients at Asan Medical Center in Seoul, Korea. Of these 708 cases, 562 cases had undergone in-house preoperative FNAC and/or ultrasoundguided CNB (US-CNB) procedures 1–3 times previously. The FNAC procedures were performed by pathologists on 371 occasions, using traditional methods with 23-gauge syringes. Two hundred and twenty-eight CNB procedures were performed by radiologists under ultrasound guidance, using a 1.1- or 1.6-cm excursion, 18-gauge, double-action, spring-activated needle (TSK Ace-cut, Create Medic, Yokohama, Japan) after administering local anesthesia with 1% lidocaine. Of these, 33 cases had undergone FNAC followed by US-CNB. No patients developed immediate or delayed complications after the procedure. We compared the diagnoses determined by preoperative FNAC without image guidance and US-CNB with the postoperative histological diagnoses. In addition, specimen adequacy, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), numbers of false-negative and -positive cases, and accurate tumor subtyping rate were analyzed. Tumor subtyping was considered accurate when one exact tumor type was diagnosed, favored, suggested, or suspected. The t test was used to evaluate differences in continuous data. The chi-squared test was used to assess the associations between categorical groups. The two-group proportion test was used to compare FNAC and CNB. All tests were two-sided, and p<.05 was considered statistically significant. Statistical analyses were performed using Stata/IC statistical software ver. 12 (StataCorp. Ltd., College Station, TX, USA).
Go to :

RESULTS
Characteristics of the examined cases
The locations of the 562 surgical cases included parotid gland (n=472), submandibular gland (n=88), and sublingual gland (n=2). Histologic diagnoses included 103 malignant and 459 benign tumors. Malignant tumors included 21 mucoepidermoid carcinomas, 17 salivary duct carcinomas, 17 carcinoma ex pleomorphic adenomas, 12 adenoid cystic carcinomas, 10 acinic cell carcinomas, 7 basal cell adenocarcinomas, 5 adenocarcinomas not otherwise specified, 3 epithelial-myoepithelial carcinomas, 3 squamous cell carcinomas, 2 oncocytic carcinomas, 1 cystadenocarcinoma, 3 malignant lymphomas, 1 rhabdomyosarcoma, and 1 undifferentiated pleomorphic sarcoma. Benign tumors included 305 pleomorphic adenomas, 96 Warthin tumors, 37 basal cell adenomas, 7 myoepitheliomas, 3 oncocytomas, 1 lymphadenoma, 5 neurogenic tumors (4 schwannomas and 1 neurofibroma), 3 vascular tumors (2 hemangiomas and 1 lymphangioma), and 2 lipomas.
When the general characteristics of the CNB and FNAC groups were compared to exclude selection bias, the proportion of malignancy, location, laterality, and multiplicity were not significantly different between the two groups (
Table 1). One significant difference was the tumor size. The average size of tumors in the FNAC group was bigger than that in the CNB group (p=.006), which can be explained by the fact that generally patients with larger palpable tumors are sent to the Pathology Department for FNAC.
Table 1.
General characteristics of salivary gland tumors according to CNB and FNAC
|
Characteristic |
|
CNB (n = 228) |
FNAC (n = 371) |
p-value |
|
Malignant:benign tumor |
|
54:174 |
62:309 |
.479 |
|
Size (mean ± SD, cm) |
|
2.57 ± 1.22 |
2.85 ± 1.21 |
.006 |
|
Site |
Parotid |
171 (75.0) |
329 (88.7) |
.150 |
|
SMG |
56 (24.6) |
40 (10.8) |
|
|
SLG |
1 (0.4) |
2 (0.5) |
|
|
Laterality |
Left |
126 (55.2) |
193 (52.0) |
.687 |
|
Right |
100 (43.9) |
175 (47.2) |
|
|
Bilateral |
2 (0.9) |
3 (0.8) |
|
|
Multiplicity |
|
14 (6.1) |
13 (3.5) |
.560 |

Specimen adequacy
Regarding the specimen adequacy of the 228 CNB specimens and 371 FNAC samples, the unsatisfactory rate tended to be lower following CNB (2.6%) than FNAC (6.2%) (
Table 2). A total of 33 cases underwent CNB after FNAC. Adenoid cystic carcinoma, salivary duct carcinoma, and oncocytoma showed high rates for multiple diagnostic procedures (3/12, 3/17, and 1/3, respectively).
Table 2.
Unsatisfactory rates and repeated diagnostic procedure rates of salivary gland tumors according to histologic diagnoses
|
Histologic diagnoses |
|
Unsatisfactory rates
|
Rates for multiple procedures |
|
CNB |
FNAC |
|
Malignancy |
ACC |
0/9 |
1/6 |
3/12 |
|
AciCC |
1/4 |
0/7 |
1/10 |
|
ANOS |
0/2 |
1/3 |
0/5 |
|
BADC |
0/2 |
1/5 |
0/7 |
|
CPA |
0/5 |
1/13 |
1/17 |
|
CystADC |
- |
1/2 |
0/1 |
|
EMC |
0/2 |
0/2 |
1/3 |
|
MEC |
1/15 |
1/7 |
1/21 |
|
OC |
- |
0/2 |
0/2 |
|
SCC |
1/3 |
0/1 |
1/3 |
|
SDC |
0/10 |
1/10 |
3/17 |
|
ML |
0/1 |
0/2 |
0/3 |
|
RMS |
0/1 |
- |
0/1 |
|
UPS |
- |
0/1 |
0/1 |
|
Subtotal |
|
3/54 (5,6) |
7/62 (11,3) |
|
|
Benign |
PA |
1/117 |
10/199 |
11/305 |
|
WT |
2/33 |
3/70 |
6/96 |
|
BA |
0/16 |
0/24 |
2/37 |
|
LA |
0/1 |
- |
0/1 |
|
ME |
0/2 |
0/6 |
1/7 |
|
Oncocytoma |
0/2 |
1/2 |
1/3 |
|
NT |
0/3 |
1/3 |
1/5 |
|
VT |
- |
0/3 |
0/3 |
|
Lipoma |
- |
1/2 |
0/2 |
|
Subtotal |
|
3/174 (1,7) |
16/309 (5,2) |
|
|
Total |
|
6/228 (2,6) |
23/371 (6,2) |
|

Accuracy
The sensitivity of detecting malignant tumors using the CNB method was significantly higher (88.2%) than that with FNAC (58.2%) (p=.006) (
Table 3). The specificity, PPV, and NPV of CNB were slightly higher than those of FNAC, without significant differences.
Table 3.
Accuracy of preoperative CNB and FNAC for diagnosing salivary gland tumors
|
Characteristic |
CNB |
FNAC |
p-value |
|
Total No. of cases |
228 |
371 |
- |
|
No. of adequate specimens, n (%) |
222 (97.4) |
348 (93.8) |
- |
|
No. of unsatisfactory specimens, n (%) |
6 (2.6) |
23 (6.2) |
.078 |
|
No. of adequate malignant cases |
51 |
55 |
- |
|
No. of preop. Dx as malignancy |
45 |
32 |
- |
|
No. of adequate benign cases |
171 |
293 |
- |
|
No. of preop. Dx as benign |
170 |
289 |
- |
|
Sensitivity (%) |
88.2 |
58.2 |
.006 |
|
Specificity (%) |
99.4 |
98.6 |
.742 |
|
Positive predictive value (%) |
97.8 |
88.9 |
.253 |
|
Negative predictive value (%) |
96.6 |
92.6 |
.121 |

False-negative and -positive cases
A total of 29 false-negative cases and 5 false-positive cases are listed in
Table 4. False-negative results by CNB were restricted to cases of basal cell adenocarcinoma, carcinoma ex pleomorphic adenoma, and epithelial-myoepithelial carcinoma, while falsenegative results by FNAC were found in a wide range of tumors including adenoid cystic carcinoma, acinic cell carcinoma, adenocarcinoma not otherwise specified, mucoepidermoid carcinoma, oncocytic carcinoma, and malignant lymphoma (
Fig. 1). No high-grade carcinomas (e.g., salivary duct carcinoma) were diagnosed as false-negatives by either method. False-positive results from neither method exhibited specific patterns; they might be the result of misinterpretation of pathologic findings, with or without artifacts.
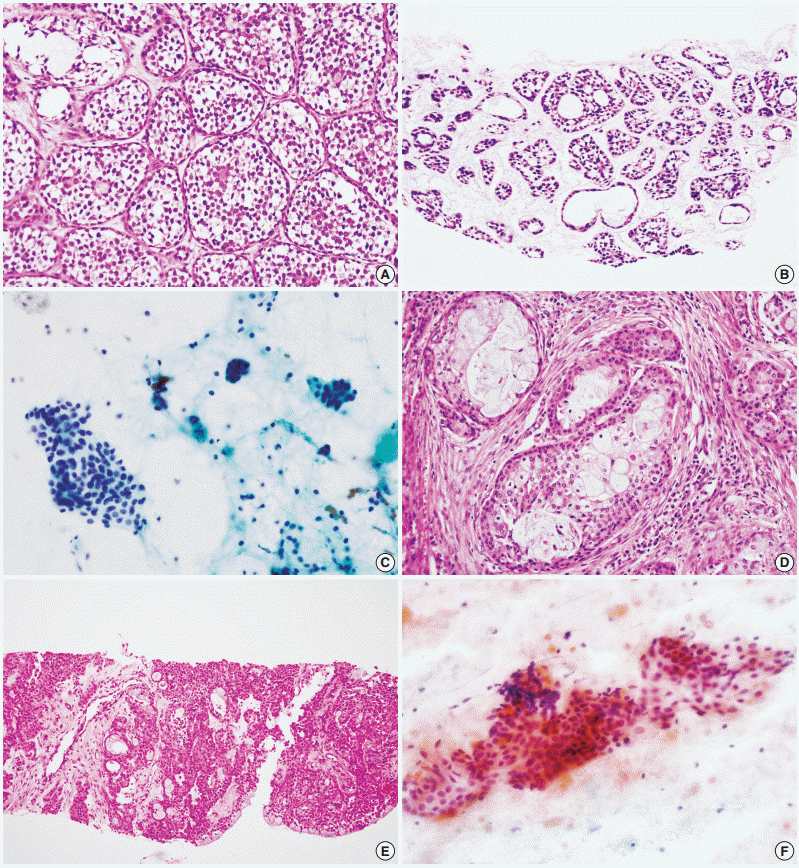 | Fig. 1.Examples of low grade carcinomas diagnosed as false-negatives by fine needle aspiration cytology. (A) Adenoid cystic carcinoma in surgical specimens. (B) Core needle biopsy shows similar architectural findings. (C) Low cellularity and lack of obvious cellular atypia in fine needle aspiration cytology were interpreted as pleomorphic adenoma. (D) Mucoepidermoid carcinoma in surgical specimens. (E) Core needle biopsy shows intermediate and mucous cells. (F) Cystic background and presence of oncocytoid components in fine needle aspiration cytology led to the misdiagnosis of Warthin tumor. 
|
Table 4.
False-negative and -positive results determined by preoperative CNB and FNAC
|
Histologic diagnoses |
CNB |
FNAC |
|
Malignancy (false-negative results) |
|
|
|
ACC |
- |
PA (n = 1), benign cyst (n = 1), mucocele (n = 1) |
|
AciCC |
- |
Oncocytoma (n = 1) |
|
ANOS |
- |
WT (n = 1) |
|
BADC |
BA (n = 2) |
BA (n = 2), benign cyst (n = 1) |
|
CPA |
PA (n = 2) |
PA (n = 7) |
|
EMC |
BA (n = 1), PA (n = 1) |
PA (n = 1), benign lesion (n = 1) |
|
MEC |
- |
PA (n = 2), benign cyst (n = 1), mucocele (n = 1) |
|
OC |
- |
Oncocytoma vs WT (n = 1) |
|
ML |
- |
Benign lymphoid lesion (n = 1) |
|
Benign (false-positive results) |
|
|
|
PA |
MEC (n = 1) |
CPA (n = 1), LG malignancy (n = 1) |
|
ME |
- |
ACC (n = 2) |

Accurate tumor subtyping
The accurate tumor subtyping rates of the salivary gland tumors were significantly higher with CNB (88.3%) than with FNAC (70.7%) (p<.001) (
Table 5). Immunohistochemical studies for tumor subtyping were performed in 11 CNB samples: CD117 in adenoid cystic carcinoma; smooth muscle actin, calponin, and p63 in pleomorphic adenoma; and S100 protein in neurogenic tumor. Tumor typing rates of benign tumors by CNB and FNAC were 91.8% and 80.5%, respectively (p=.003). For malignant tumors, accurate tumor subtyping was achieved in 39 of 51 CNB cases (76.5%), but in only 10 of 55 FNAC cases (18.2%) (p=.002). For a few special entities, both methods faced diagnostic difficulties. Since the diagnosis of basal cell adenocarcinoma and oncocytic carcinoma requires extracapsular invasion by definition, none of these cases could be diagnosed using either CNB or FNAC (
Fig. 2). Similarly, the diagnosis of carcinoma ex pleomorphic adenoma was not possible without concomitant carcinoma and pleomorphic adenoma components, even by CNB. The diagnosis of epithelial-myoepithelial carcinoma was difficult by either method, most likely due to its resemblance to pleomorphic adenoma, its low-grade nature, and a low index of suspicion (
Fig. 2).
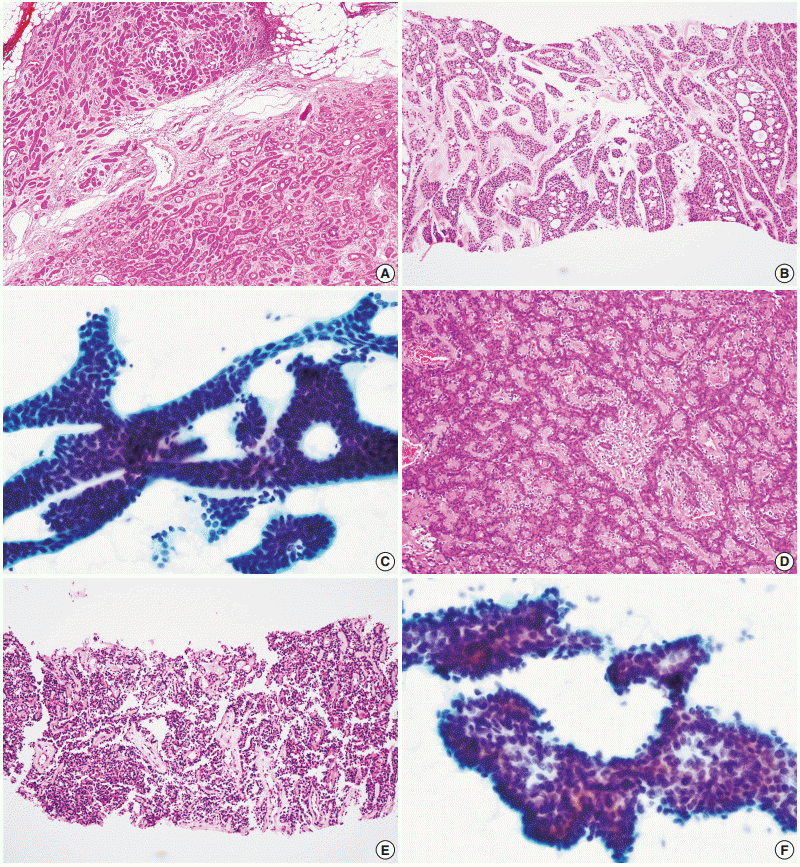 | Fig. 2.Difficult samples for both core needle biopsy and fine needle aspiration. (A) Surgical specimen of basal cell adenocarcinoma shows extracapsular invasion which cannot be confirmed in core needle biopsy (B) or fine needle aspiration cytology (C). (D) Epithelial-myoepithelial structures of epithelial-myoepithelial carcinoma can be mistaken for those of pleomorphic adenoma in both core needle biopsy (E) and fine needle aspiration cytology (F), because of the lack of obvious cellular atypia. 
|
Table 5.
Accurate tumor subtyping rates of salivary gland tumors determined by preoperative CNB and FNAC
|
Histologic diagnoses |
|
CNB |
FNAC |
p-value |
|
Malignancy |
ACC |
9/9 |
2/5 |
|
|
AciCC |
3/3 |
4/7 |
|
|
ANOS |
2/2 |
0/2 |
|
|
BADC |
0/2 |
0/4 |
|
|
CPA |
2/5 |
0/12 |
|
|
CystADC |
- |
0/1 |
|
|
EMC |
0/2 |
0/2 |
|
|
MEC |
12/14 |
2/6 |
|
|
OC |
- |
0/2 |
|
|
SCC |
2/2 |
0/1 |
|
|
SDC |
7/10 |
1/9 |
|
|
ML |
1/1 |
1/2 |
|
|
RMS |
1/1 |
- |
|
|
UPS |
- |
0/2 |
|
|
Subtotal |
|
39/51 (76.5) |
10/55 (18.2) |
.002 |
|
Benign |
PA |
111/116 |
170/189 |
|
|
WT |
31/31 |
53/66 |
|
|
BA |
12/16 |
10/24 |
|
|
LA |
0/1 |
- |
|
|
ME |
0/2 |
2/6 |
|
|
Oncocytoma |
1/2 |
0/2 |
|
|
NT |
2/3 |
1/2 |
|
|
VT |
- |
0/3 |
|
|
Lipoma |
- |
0/1 |
|
|
Subtotal |
|
157/171 (91.8) |
236/293 (80.5) |
.003 |
|
Total |
|
196/222 (88.3) |
246/348 (70.7) |
<.001 |

Go to :

DISCUSSION
In 1999, Buckland
et al. [
13] introduced US-CNB using an 18- gauge needle, instead of fine needle aspiration using a 23-gauge needle, to evaluate salivary gland lesions. They reported satisfactory results based on their experiences of diagnosing and treating parotid gland masses in up to 220 patients [
14-
17]. The technique was soon adopted by other groups as well; small series of CNB results for salivary gland tumors have been reported from several countries, including the UK, Taiwan, Japan, and Germany [
11,
12,
18-
20].
Our current study of 228 CNB and 371 FNAC procedures demonstrates the superiority of CNB over FNAC for diagnosing salivary gland tumors in terms of adequacy (97.4% vs 93.8%), sensitivity (88.2% vs 58.2%), specificity (99.4% vs 98.6%), PPV (97.8% vs 88.9%), NPV (96.6% vs 92.6%), and accurate tumor subtyping (88.3% vs 70.7%). Among these measures, differences in the sensitivity and tumor typing rate were statistically significant. These results are based on the histological confirmation of surgically treated cases. Although this type of design tends to lead to verification bias [
21], we did not include follow-up cases because our aims were to compare the accuracy of the two tests for specific diagnoses. As a result, the sensitivities of both methods may have been overestimated due to verification bias [
21]. Even if the bias affected both methods, the sensitivity of CNB appears to be markedly improved, which can be attributed to the ability to recognize tumor structures by histological examination in CNB and not just cellular morphology alone as in FNAC.
The diversity and rarity of salivary gland carcinomas tend to provide diagnostic challenges for pathologists. Diagnosis of malignancy can be difficult when the cells in question pose no significant cytologic atypia. In addition, pathologists’ experience and knowledge can affect the accuracy of FNAC. In our current study, no high-grade carcinomas, including salivary duct carcinoma and squamous cell carcinoma, were diagnosed as falsenegatives using FNAC; however, low-grade carcinomas, including adenoid cystic carcinoma, acinic cell carcinoma, mucoepidermoid carcinoma, epithelial-myoepithelial carcinoma, and adenocarcinoma not otherwise specified, were occasionally misinterpreted as benign lesions. The PPV and NPV, which are not affected by verification bias, were also higher in CNB than in FNAC, though the differences were not statistically significant.
The difficulties of diagnosing basal cell adenocarcinoma, oncocytic carcinoma, and carcinoma ex pleomorphic adenoma apply to not only FNAC, but also to CNB when invasive and/or malignant foci are not sampled. For example, we misinterpreted two epithelial-myoepithelial carcinomas as pleomorphic adenoma in one and basal cell adenoma in the other, and one pleomorphic adenoma as mucoepidermoid carcinoma in CNB. Salivary gland tumors are diverse and also analogous with specific architectural patterns such as epithelial-myoepithelial structures which are present in various benign and malignant tumors. Interpreting a limited number of cores can be difficult, even for experienced pathologists. Nonetheless, accurate tumor subtyping rates were generally higher for CNB than for FNAC (88.3% vs 70.7%). In particular, malignant tumors were more often accurately classified using CNB than FNAC (76.5% vs 18.2%) in comparison to the benign tumors (91.8% vs 80.5%). Both highand low-grade carcinomas could be more specifically diagnosed by CNB than by FNAC.
Some clinicians prefer FNAC because it has technical advantages such as simplicity of the procedure, safety, cost-effectiveness, and the lack of need for ultrasound assistance. However, the CNB procedure is generally well tolerated under local anesthesia, and the actual complication rate of CNB appears to be far less than expected. The major complications of salivary gland biopsy include facial nerve injury and tumor seeding along the biopsy track. However, experienced radiologists can avoid facial nerve injury by tracing the main intraparotid vessels or the parotid duct, which can be easily identified on ultrasound [
14,
18]. Tumor seeding was once considered a significant complication when performing large needle biopsy on cancers, and the needle diameter and number of passes are assumed to be related to this risk [
22]. However, such evidence is lacking in the case of salivary gland tumors. Two cases of tumor seeding following needle biopsy of the salivary gland using 14–16-gauge needles have been previously reported, but a few reports of tumor seeding following FNAC have also been reported more recently [
23,
24]. However low the risk, some authors have suggested surgical removal of the biopsy track at the time of surgery [
12,
25].
No studies on the use of 18-gauge CNB to assess the salivary glands, including our present series, have reported these major complications. The minor complications that have been reported following salivary gland CNB include subclinical hematoma [
11,
12,
14-
16,
18,
20], temporary facial weakness after local anesthesia [
11], and the formation of salivary fistulas [
17]. Fistula developed after post-biopsy acute parotitis and did not present with tumor seeding [
17]. Increased awareness of this rare complication would help provide better patient care and follow-up.
In conclusion, CNB is an accurate and safe method for diagnosing salivary gland lesions, and provides significant superiority in accurate tumor subtyping in comparison to FNAC. We recommend CNB as the primary diagnostic tool for preoperatively evaluating salivary gland masses, especially when malignancy is suspected.
Go to :







 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download