Abstract
Background:
Follicular thyroid carcinoma (FTC) is the second most common thyroid malignancy and its differential diagnosis includes follicular adenoma (FA) and adenomatous goiter (AG). Several ancillary markers have been suggested to aid in the diagnosis of FTC, but the successful use of these methods still needs to be validated.
Methods:
In the present study, we verified the immunoexpression of HMGA2, CEACAM6, survivin, and SFN/14-3-3 δ in lesions including 41 AGs, 72 FAs, and 79 FTCs. We evaluated their diagnostic usefulness, combined with galectin 3, Hector Battifora mesothelial 1 (HBME1), cytokeratin 19, and cyclin D1, in diagnosing FTC.
Results:
The expressions of HBME1 (65.8%) and HMGA2 (55.7%) were significantly higher in FTCs than in FAs and AGs (p<.001 and p=.005, respectively). HBME1 was the only marker that was more frequently expressed in FTCs than in FAs (p=.021) and it was more frequently expressed in follicular neoplasms than in AGs (p<.001). Among the novel markers, the combination of HMGA2 and HBME1 showed the highest sensitivity (72.2%) and specificity (76.1%) for diagnosing FTC. CEACAM6, survivin, and SFN/14-3-3 δ were barely expressed in most cases.
Follicular thyroid carcinoma (FTC) is the second most common thyroid malignancy with a reported incidence of 5%–20% [1]. Its preoperative diagnosis is not possible, as FTC can only be diagnosed in a surgically-resected specimen [2,3]. However, the differential diagnosis between FTC, follicular adenoma (FA), and adenomatous goiter (AG) is often difficult, even in resected lesions. Occasionally AG has a fibrous capsule and solid proliferation of follicles, requiring detailed microscopic examination for its diagnosis [4,5]. The distinction between benign and malignant follicular-patterned lesions solely depends on the presence of capsular and/or vascular invasion [4-7]. Therefore, all patients with follicular neoplasm (FN) diagnosed with fine needle aspiration are recommended to undergo a thyroid lobectomy for histologic confirmation [2]. If the tumor is diagnosed as FTC, additional resection of the remaining thyroid or lymph node is required.
Previous studies have reported differential expression of several genes in malignant neoplasms, and the use of some of those markers for differential diagnosis has been subsequently validated in formalin-fixed paraffin-embedded (FFPE) tissues [8-15]. DDIT3, ARG2, ITM1, and C1orf24 have been tested as additional diagnostic tools for distinguishing FTC from FA, but none have been proven to be reliable markers [16,17]. CEACAM6, HMGA2, and SFN/14-3-3 δ (stratifin) need to be validated using FFPE thyroid tissues. Additionally, it has been suggested that the expression of survivin is higher in FTC than in FA, but the number of cases was limited in the study (FTC, 11 cases) [11]. Galectin 3 (Gal-3), Hector Battifora mesothelial 1 (HBME1), cytokeratin 19 (CK19), and cyclin D1, all of which are well-established diagnostic markers for papillary thyroid carcinoma (PTC), have also been used in differentiating FTC from other benign follicular lesions but the results are controversial [15,18-20].
In the present study, we evaluated the immunohistochemical expression of HMGA2, CEACAM6, survivin, SFN/14-3-3 δ, Gal-3, HBME1, CK19, and cyclin D1 in lesions including 41 AGs, 72 FAs, and 79 FTCs. We also evaluated their diagnostic usefulness in differentiating FTC.
Formalin-fixed, paraffin-embedded thyroid tissue blocks were retrieved from the archive maintained at the Department of Pathology, Seoul National University Hospital, from 2001 to 2013. A total of 192 cases of thyroid lesions consisting of 41 AGs, 72 FAs, and 79 FTCs were identified, and representative tissue blocks were obtained for all lesions. All cases were surgically resected. The hematoxylin and eosin–stained slides were reviewed in each case to confirm the original diagnosis using the strict criteria of capsular invasion and vascular invasion defined by two pathologists (M.H.J. and H.S.M.). AG cases were selected from the archives from 2001 to 2007, with identification of the follow-up records for confirming their benign nature.
Tissue microarrays were constructed for immunohistochemical staining. A single, large tissue core (4.0 mm in diameter) was obtained from the most representative area of individual cases. Additionally, 74 cores of normal thyroid tissue from each matched thyroid lesion were included for negative controls. This study was approved by the Institutional Review Board of Seoul National University Hospital (E-1302-023-462).
Immunohistochemistry was performed on 4-µm-thick sections of tissue microarray blocks that included 192 surgicallyremoved samples. Tissue sections were deparaffinized and rehydrated following standard procedures. Heat-induced antigen retrieval was carried out and sections were incubated with primary antibodies for 32 minutes at 37°C at a dilution of 1:50 for HBME1 and cyclin D1, 1:100 for Gal-3 and SFN/14-3-3 δ, 1:200 for CK19, HMGA2 and CEACAM6, and 1:600 for survivin. Monoclonal antibodies were used for Gal-3 (clone 9C4, Novocastra, Newcastle, United Kingdom), HBME1 (clone HBME-1, Dako, Carpinteria, CA, USA), CK19 (clone RCK108, Dako), cyclin D1 (clone SP4, Thermo Fisher Scientific, Waltham, MA, USA), CEACAM6 (clone 9A6, Abcam, Cambridge, MA, USA) and SFN/14-3-3 δ (clone 5D7, Santacruz, Dalla, TX, USA). Polyclonal antibodies were used for HMGA2 (Biocheck, Foster city, CA, USA) and survivin (Novus Biologicals, Littleton, CO, USA). All immunohistochemical staining was carried out in a BenchMark XT autostainer (Ventana Medical Systems, Tucson, AZ, USA) using the DAB detection kit (Ventana Medical Systems).
The immunohistochemical staining of tissue microarrays was evaluated by two pathologists (M.H.J. and H.S.M.). The immunoreactivity was scored for Gal-3, HBME1, CK19, cyclin D1, HMGA2, CEACAM6, survivin, and SFN/14-3-3 δ by categorizing methods based on the percentage of positive cells: 0 (less than 10%), 1 (10%–25%), 2 (26%–50%), and 3 (more than 50%). In Gal-3, CK19, survivin, and SFN/14-3-3 δ, cytoplasmic staining was considered as positive immunoreactivity. Membranous staining was regarded as positive for HBME1 and CEACAM6, and nuclear expression was regarded as positive for cyclin D1 and HMGA2.
The data was analyzed using SPSS ver. 21.0.0 for Windows (SPSS Inc., Chicago, IL, USA). The χ2 test or Fisher exact test was used to compare the expression of markers between different diagnostic subgroups. A p-value of <.05 was considered statistically significant. Sensitivity, specificity, and diagnostic accuracy were calculated using standard formulae for each marker individually, using histological diagnosis as the gold standard.
The whole series of samples was obtained from 36 males and 156 females, with a median age of 46 years (range, 9 to 76 years). The mean size of FAs and FTCs was 1.83 cm (range, 1.4 to 4.7 cm) and 3.7 cm (range, 0.8 to 7.3 cm), respectively. The FTC series included samples from 17 males and 61 females, with a median age of 42 years (range, 9 to 76 years). There were 19 cases with vascular invasion. There was only one case that metastasized to lung, and one case recurred in neck soft tissue 15 months after a total thyroidectomy. Unfortunately, we could not obtain the clinicopathologic information of the primary tumor of the metastatic FTC. The clinicopathologic characteristics of the 78 primary FTCs are summarized in Table 1.
Most markers, including Gal-3, HBME1, cyclin D1, HMGA2, CEACAM6, survivin, and SNF/14-3-3 δ, were not expressed in the 74 normal thyroid tissues. On the contrary, CK19 was expressed as grade 1 (10%–25%) or 2 (26%–50%) in 31.1% of the normal thyroid tissues.
In the AG group (n=41), most cases showed negative or limited immunoreactivity (less than grade 1, ≤25%) for all eight markers. Gal-3 was expressed in one case, but it showed relatively diffuse expression (grade 2, 26%–50%). HBME1, CK19, and cyclin D1 were expressed in only a few cases, showing grade 2 or 3 (grade 2: HBME1, 2/41; CK19, 3/41; cyclin D1, 3/41; grade 3: HBME1, 2/41; CK19, 0/41; cyclin D1, 0/41). However, HMGA2 was expressed in more cases, showing grade 2 (6/41) and grade 3 (4/41). All cases with expression of HBME1 (3/41) and HMGA2 (6/41) exhibited a characteristic feature of microfollicular proliferation of various extents, reminiscent of FN, and showed HBME1 and HMGA2 positivity in this area.
In the FA group (n=72), HBME1, cyclin D1, and HMGA2 were expressed in more than 25% of tumor cells (grade 2: HBME1, 12/72; cyclin D1, 24/72; HMGA2, 12/72; grade 3: HBME1, 22/72; cyclin D1, 14/72; HMGA2, 18/72). There was no diffuse staining of CEACAM6, survivin, and SNF/14-3-3 δ. CK19 was expressed only in four cases, either as grade 2 (3/72) or grade 3 (1/72).
The expression of HBME1 and HMGA2 was the highest in the FTC group, followed by that of cyclin D1, which was expressed at a similar frequency with the FA group. However, HBME1 and HMGA2 expression were not significantly different between FTCs without vascular invasion and FTCs with vascular invasion (p=.382 and p=.418, respectively). The frequency of CK19 expression was higher in the FTC group than in the FA group, but the difference was not statistically significant (p=.133). There was no diffuse staining of CEACAM6, survivin, or SNF/14-3-3 δ (Table 2).
Overall, among the novel markers, SFN/14-3-3 δ and CEACAM6 were not expressed in any of the subgroups, and survivin was only expressed in a small percentage of lesions (grade 0, less than 10%) in 14/97 FTCs. Therefore, these three markers were not helpful in distinguishing the diagnostic subgroups. In contrast, HMGA1 was significantly expressed in FTC and FA cases. The represented immunohistochemical expression of all markers is shown in Fig. 1.
Next, we compared the expression of each marker between the diagnostic subgroups. As survivin, CEACAM6 and SNF/14- 3-3 δ were expressed in only a few cases or were not expressed at all, they were excluded from the statistical analysis for the evaluation of diagnostic utilities.
HBME1 was the only marker that showed differential expression frequency between FTC and FA (p=.021) (Table 2). However, it was only expressed in 52 of 79 FTCs (sensitivity, 65.8%) and its specificity remained as 52.8%. When comparing malignant and benign lesions (FTC vs FA and AG), the expression of HBME1 (p<.001) and HMGA2 (p=.005) was significantly different. In the diagnosis of malignancy, HBME1 showed a slightly better sensitivity (65.8%) and specificity (66.4%) than HMGA2 (sensitivity, 55.7%; specificity, 64.6%).
Additionally, we calculated the sensitivity, specificity, and diagnostic accuracy of the combination of HBME1 and HMGA2 (Table 3). The combined expression of HBME1 or HMGA2 reached the highest sensitivity (72.2%), but the specificity (54.9%) and the diagnostic accuracy (62.0%) were similar or only slightly higher than those of the single markers. The simultaneous expression of HBME1 and HMGA2 increased the specificity up to 76.1%, but its sensitivity (49.4%) was poor.
There were no differences when comparing the diagnostic utility of the combination of HBME1, cyclin D1 and HMGA2 with those of each single marker in neoplastic lesions (FN including FTC and FA vs AG) (Table 4). As a single marker, HBME1 showed the highest sensitivity (57.0%), and both HBME1 and cyclin D1 showed the highest specificity (90.2%). When more than one marker was expressed among HBME1, cyclin D1, and HMGA2, the sensitivity reached 80.9%, but the specificity decreased.
Until now, the entire histologic examination of the fibrous capsule and vasculature after surgery has been the only way to precisely diagnose FTC. Thus, a preoperative diagnosis of FTC is needed in making an accurate preoperative plan and avoiding unnecessary surgery. In this study, we validated the diagnostic utility of HMGA2, CEACAM6, survivin, and SNF/14-3-3 δ with several known markers for distinguishing FTCs, expecting to find out a powerful diagnostic panel.
HMGA2, CEACAM6, and SFN/14-3-3 δ were identified as promising molecular markers that were differentially expressed between benign and malignant thyroid tumors in a previous report by Prasad et al. [14]. In the immunohistochemical study, HMGA2 and SFN/14-3-3 δ were highly expressed in malignant tumors (HMGA2, p<.001, area under the curve [AUC]= 0.84; SFN/14-3-3 δ, p<.001, AUC=0.83). However, CEACAM6 did not show significantly different immunoreactivity [8]. Belge et al. [12] suggested that quantifying HMGA2 expression by reverse transcription polymerase chain reaction had a high potential to improve the diagnosis of FNs with a sensitivity of 95.9% and a specificity of 93.9%. In our study, the sensitivity and specificity of HMGA2 for FTC were 55.7% and 64.6%, respectively. However, CEACAM6 was only expressed in infiltrated inflammatory cells (Fig. 1). SFN/14-3-3 δ was only validated in 14 FTC cases in a previous study and thus, the results needed to be verified. Interestingly, its expression was specific for PTC [8,21]. Nevertheless, our study suggested that SFN/ 14-3-3 δ was not an applicable marker for FTC and FN.
Haghpanah et al. [11] reported that the cytoplasmic expression of survivin was significantly higher in FTCs than in FAs (p<.005), with a high odds ratio (odds ratio, 21.4), but the number of cases was limited (11 FTC cases, 23 FA cases). Recently, Kim et al. [22] observed the immunoexpression of survivin in 13/57 FTCs but also in 21/58 FA cases. However, survivin was only expressed in a small proportion (<10%) of FTCs (14/ 97) in our study (Fig. 1).
Among the well-known markers for PTC that we tested in this study (Gal-3, CK19, and HBME1), HBME1 was the only one expressed at significantly higher levels in the FTC group compared to other groups. The use of HBME1 as a marker of FTC is controversial, and its low specificity has not allowed for the differential diagnosis of FTCs in previous reports [23,24]. Our study yielded a similar result, showing that although the expression of HBME1 was significantly higher in the FTC group, it was expressed in almost half of FAs as well. However, it could differentiate FNs from AGs (p<.001), showing positivity in only 4/41 AG cases.
Lastly, our results suggest that the combination use of HBME1 and HMGA2 can be beneficial in the differential diagnosis of FTC. Both markers showed significantly increased expression in FTCs when used alone. When either HBME1 or HMGA2 alone, or both HBME1 and HMGA2 were expressed in lesions, the sensitivity for detecting FTC reached 72.7%. When both markers were simultaneously positive, the specificity reached 76.1%. Therefore, the concurrent use of HBME1 and HMGA2 may be more beneficial than the single use, but it requires a more sophisticated interpretation for FTC diagnosis.
In summary, among all the novel immunohistochemical markers that we tested, HMGA2 was expressed at a higher level in FTCs than in FAs or AGs, but its overall sensitivity was slightly lower than that of HBME1. However, the combination of HMGA2 and HBME1 may be beneficial in differentiating FTCs, as it increased the sensitivity and the specificity for FTCs. Although survivin, CEACAM6, and SFN/14-3-3 δ were initially promising in differentiating malignancy, our results showed that only HMGA2 could help in the diagnosis of FTC.
REFERENCES
1. Kapur U, Wojcik EM. Follicular neoplasm of the thyroid: vanishing cytologic diagnosis? Diagn Cytopathol. 2007; 35:525–8.
2. Wiseman SM, Baliski C, Irvine R, et al. Hemithyroidectomy: the optimal initial surgical approach for individuals undergoing surgery for a cytological diagnosis of follicular neoplasm. Ann Surg Oncol. 2006; 13:425–32.

3. McHenry CR, Phitayakorn R. Follicular adenoma and carcinoma of the thyroid gland. Oncologist. 2011; 16:585–93.

4. LiVolsi VA, Baloch ZW. Follicular-patterned tumors of the thyroid: the battle of benign vs. malignant vs. so-called uncertain. Endocr Pathol. 2011; 22:184–9.

5. Sobrinho-Simões M, Eloy C, Magalhães J, Lobo C, Amaro T. Follicular thyroid carcinoma. Mod Pathol. 2011; 24 Suppl 2:S10–8.

7. Baloch ZW, LiVolsi VA. Our approach to follicular-patterned lesions of the thyroid. J Clin Pathol. 2007; 60:244–50.

8. Prasad NB, Kowalski J, Tsai HL, et al. Three-gene molecular diagnostic model for thyroid cancer. Thyroid. 2012; 22:275–84.

9. Cerutti JM, Delcelo R, Amadei MJ, et al. A preoperative diagnostic test that distinguishes benign from malignant thyroid carcinoma based on gene expression. J Clin Invest. 2004; 113:1234–42.

10. Cerutti JM, Latini FR, Nakabashi C, et al. Diagnosis of suspicious thyroid nodules using four protein biomarkers. Clin Cancer Res. 2006; 12(11 Pt 1):3311–8.

11. Haghpanah V, Shooshtarizadeh P, Heshmat R, Larijani B, Tavangar SM. Immunohistochemical analysis of survivin expression in thyroid follicular adenoma and carcinoma. Appl Immunohistochem Mol Morphol. 2006; 14:422–5.

12. Belge G, Meyer A, Klemke M, et al. Upregulation of HMGA2 in thyroid carcinomas: a novel molecular marker to distinguish between benign and malignant follicular neoplasias. Genes Chromosomes Cancer. 2008; 47:56–63.
13. Chiappetta G, Ferraro A, Vuttariello E, et al. HMGA2 mRNA expression correlates with the malignant phenotype in human thyroid neoplasias. Eur J Cancer. 2008; 44:1015–21.

14. Prasad NB, Somervell H, Tufano RP, et al. Identification of genes differentially expressed in benign versus malignant thyroid tumors. Clin Cancer Res. 2008; 14:3327–37.

15. Paunovic I, Isic T, Havelka M, Tatic S, Cvejic D, Savin S. Combined immunohistochemistry for thyroid peroxidase, galectin-3, CK19 and HBME-1 in differential diagnosis of thyroid tumors. APMIS. 2012; 120:368–79.

16. Bryson PC, Shores CG, Hart C, et al. Immunohistochemical distinction of follicular thyroid adenomas and follicular carcinomas. Arch Otolaryngol Head Neck Surg. 2008; 134:581–6.

17. Sigstad E, Paus E, Bjøro T, et al. The new molecular markers DDIT3, STT3A, ARG2 and FAM129A are not useful in diagnosing thyroid follicular tumors. Mod Pathol. 2012; 25:537–47.

18. de Matos PS, Ferreira AP, de Oliveira Facuri F, Assumpção LV, Metze K, Ward LS. Usefulness of HBME-1, cytokeratin 19 and galectin-3 immunostaining in the diagnosis of thyroid malignancy. Histopathology. 2005; 47:391–401.

19. Prasad ML, Pellegata NS, Huang Y, Nagaraja HN, de la Chapelle A, Kloos RT. Galectin-3, fibronectin-1, CITED-1, HBME1 and cytokeratin-19 immunohistochemistry is useful for the differential diagnosis of thyroid tumors. Mod Pathol. 2005; 18:48–57.

20. Papale F, Cafiero G, Grimaldi A, et al. Galectin-3 expression in thyroid fine needle cytology (t-FNAC) uncertain cases: validation of molecular markers and technology innovation. J Cell Physiol. 2013; 228:968–74.

21. Lal G, Padmanabha L, Provenzano M, Fitzgerald M, Weydert J, Domann FE. Regulation of 14-3-3sigma expression in human thyroid carcinoma is epigenetically regulated by aberrant cytosine methylation. Cancer Lett. 2008; 267:165–74.
22. Kim YA, Chang M, Park YJ, Kim JE. Detection of survivin and COX- 2 in thyroid carcinoma: anaplastic carcinoma shows overexpression of nuclear survivin and low COX-2 expression. Korean J Pathol. 2012; 46:55–60.
Fig. 1.
Representative immunohistochemical results in follicular thyroid carcinoma (FTC). Galectin 3 (A), cytokeratin 19 (C), survivin (F), CEACAM6 (G), and SFN/14-3-3 δ (H) are only occasionally expressed or not expressed. However, Hector Battifora mesothelial 1 (B), HMGA2 (E), and cyclin D1 (D) show diffuse positivity in many FTC cases.
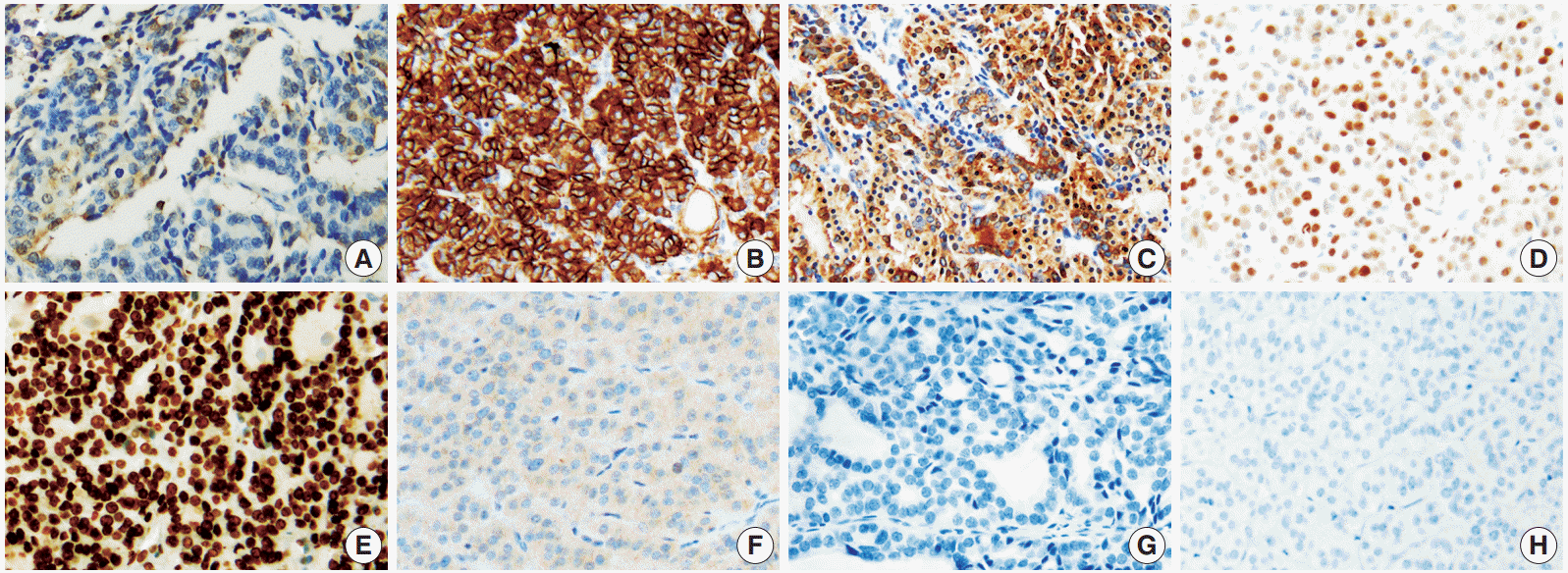
Table 1.
Clinicopathologic characteristics of 78 FTCs
Table 2.
Immunohistochemistry in AG, FA, FTC and its association with histologic diagnosis
Table 3.
Diagnostic values of HBME1/HMGA2 for malignancy (FTC vs FA and AG)
Table 4.
Diagnostic values of markers for FN (FTC and FA vs AG)




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download