Until now, fewer than 60 cases of tumors originating from the seminal vesicle have been reported. Most of them were adenocarcinoma, cystadenoma, and benign mesenchymal tumor [1]. Among them, only three cases in the English literature have been reported as squamous cell carcinoma [2-4]. Although all three cases had a prolonged history of stone formation or chronic inflammation, none of them were associated with congenital malformation of the urogenital system such as Zinner syndrome. Zinner syndrome is a rare Müllerian duct abnormality consisting of unilateral renal agenesis, ipsilateral seminal vesicle cyst, and ejaculatory duct obstruction. Herein, we report a 41-year-old male with Zinner syndrome, who developed a poorly differentiated squamous cell carcinoma of the seminal vesicle as a result of prolonged inflammation.
A 41-year-old male was admitted to our hospital due to gross hematuria for two and a half months. Twelve years previous, the patient underwent transurethral resection, suprapubic cystostomy, and urethral sounds to cure a right seminal vesicle cyst with multiple stones and obstruction of the right ejaculatory duct. At that time, a biopsy diagnosed the seminal vesicle cyst as an epidermal cyst, a benign cyst lined with a thin layer of squamous epithelium. A week after his current admission, perineal and scrotal pains newly developed. Digital rectal examination detected tenderness with a hard and highly elevated posterior prostate compressing the rectal wall. No prostate-specific antigen elevation was detected and urine cytology was negative for malignant cells. Computed tomography (CT) urography revealed hypoplastic change in the right kidney, a 4.9-cm-sized right seminal vesicle cyst with a thickened wall, and benign prostate hyperplasia (Fig. 1A). The patient was diagnosed with Zinner syndrome with a seminal vesicle cyst. Transrectal sonography detected a 3.8-cm-sized hypoechoic lesion at the left transitional zone of the prostate showing a bulging contour and prominent vascularity, which favored chronic prostatitis over malignancy. Treatment included not only palliative medication but also aggressive procedures such as nerve blocking for pain control. Six months after the initial onset of gross hematuria, another transrectal biopsy was done and the specimen was pathologically diagnosed as poorly differentiated carcinoma. CT urography and magnetic resonance imaging demonstrated a bulging mass at the left prostate gland, which had increased in size compared with images taken three months previous (Fig. 1B). The mass showed signs of internal necrosis and anorectal adhesion, but did not show anorectal invasion. A 4.7-cm-sized seminal vesicle cyst at the right side and necrotic lymph nodes at the left external iliac area were also observed. Through surgery, the urinary bladder, prostate, and bilateral seminal vesicles were removed en bloc. On gross examination, the specimen consisted of an 11.0×6.0×5.0-cm-sized multinodular mass with a tan-white cut surface accompanied by hemorrhage and necrosis. Adjacent to the tumor, the seminal vesicle cyst had a smooth mucosa measuring 5.0×3.0 cm. Under the microscope, squamous metaplasia was observed in the seminal vesicle cyst lining (Fig. 2A). Microscopically observed, the tumor was a poorly differentiated carcinoma (Fig. 2B). Immunohistochemical analysis of the tumor was positive for p63 (Fig. 3A) and negative for cytokeratin 7 (Fig. 3B), cytokeratin 20 (Fig. 3C), and carcinoembryonic antigen (Fig. 3D). Although the specimen displayed some immunoactivity for vimentin (Fig. 3E), the staining was positive in macrophages, as reported in murine seminal vesicle carcinoma [5]. Histochemical studies (periodic acid–Schiff [PAS] and mucicarmine) were positive for mucin (Fig. 3F). The tumor was pathologically diagnosed as poorly differentiated squamous cell carcinoma arising from the seminal vesicle cyst. The involvement of carcinoma was detected in rectal and right ureter tissues, but no lymph node metastasis was observed. The postoperative course had no further events. The patient was discharged and scheduled for adjuvant concurrent chemoradiation therapy.
Our patient suffered from Zinner syndrome, a male counterpart of Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome which occurs in females. MRKH syndrome is reportedly associated with ovarian cancer and renal cell carcinoma in the genitourinary tract [6,7]. Two cases of adenocarcinoma arising from a seminal vesicle cyst in Zinner syndrome patients have been previously reported [8,9]. Table 1 summarizes those cases as well as the present case.
In the current case, no diagnostic tool was able to detecting the hidden malignancy until the inflammation subsided. Moreover, poorly differentiated and wide-spread features of the malignancy made it difficult to determine the exact origin of the tumor before surgery. In this case, squamous metaplasia was pathologically proven 12 years prior to the discovery of the malignancy, which clarifies the origin of the tumor. By contrast, in all three previously reported cases of squamous carcinoma arising from the seminal vesicle, squamous metaplastic foci were diagnosed simultaneously with the carcinoma [2-4]. Although tumor cells in the present case were positive for mucin in PAS and mucicarmine staining, it is not uncommon for a squamous cell carcinoma to express mucin content [10].
To the best of our knowledge, this is the first report of a squamous cell carcinoma of the seminal vesicle developed in a Zinner syndrome patient. Our case indicates that long-term observation and thorough evaluation are mandatory for patients with Zinner syndrome expressing nonspecific but rapid and progressive urogenital symptoms.
REFERENCES
1. Lorber G, Pizov G, Gofrit ON, Pode D. Seminal vesicle cystadenoma: a rare clinical perspective. Eur Urol. 2011; 60:388–91.

2. Tabata K, Irie A, Ishii D, Yanagisawa N, Iwamura M, Baba S. Primary squamous cell carcinoma of the seminal vesicle. Urology. 2002; 59:445.

3. Yanagisawa N, Saegusa M, Yoshida T, Okayasu I. Squamous cell carcinoma arising from a seminal vesicular cyst: possible relationship between chronic inflammation and tumor development. Pathol Int. 2002; 52:249–53.

4. Wang J, Yue X, Zhao R, Cheng B, Wazir R, Wang K. Primary squamous cell carcinoma of seminal vesicle: an extremely rare case report with literature review. Int Urol Nephrol. 2013; 45:135–8.

5. Shoda T, Mitsumori K, Imazawa T, et al. A spontaneous seminal vesicle adenocarcinoma in an aged F344 rat. Toxicol Pathol. 1998; 26:448–51.

6. Ghirardini G, Magnani A. Mayer-Rokitansky-Küster-Hauser syndrome and ovarian cancer: report of a case. Clin Exp Obstet Gynecol. 1995; 22:247–8.
7. Mermerkaya M, Burgu B, Hamidi N, et al. Mayer-Rokitansky-Küster-Hauser syndrome accompanied by renal cell carcinoma: a case report. J Pediatr Hematol Oncol. 2013; 35:e309–10.
8. Okada Y, Tanaka H, Takeuchi H, Yoshida O. Papillary adenocarcinoma in a seminal vesicle cyst associated with ipsilateral renal agenesis: a case report. J Urol. 1992; 148:1543–5.

Fig. 1.
Computed tomography urography. (A) Images taken three months before the surgery demonstrate a 4.9-cm-sized right seminal vesicle cyst (arrowhead) and an exophytic tumor measuring up to 3 cm (arrow). (B) Images taken three months later show a 4.7-cm-sized seminal vesicle cyst (arrowhead) with an enlarged mass (arrow) bulging to the left prostate gland (6.1 cm).
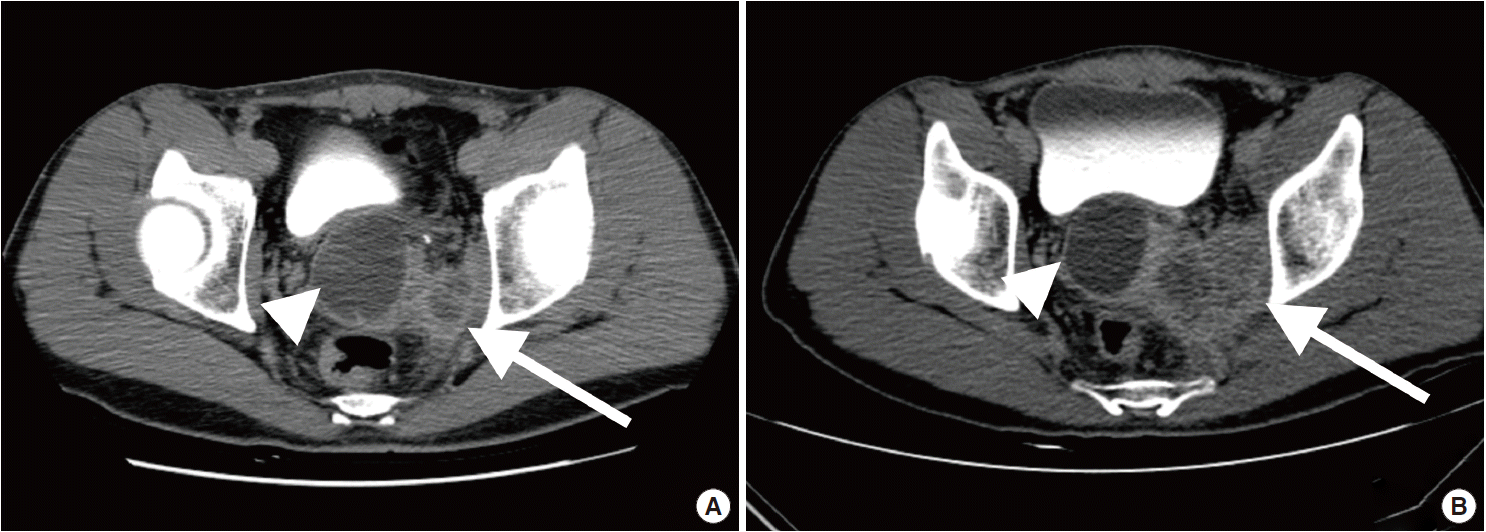
Fig. 2.
Histopathologic findings of the right seminal vesicle cyst. The specimen shows squamous metaplasia (insert), glandular tissues (A), and a poorly differentiated carcinoma (B).
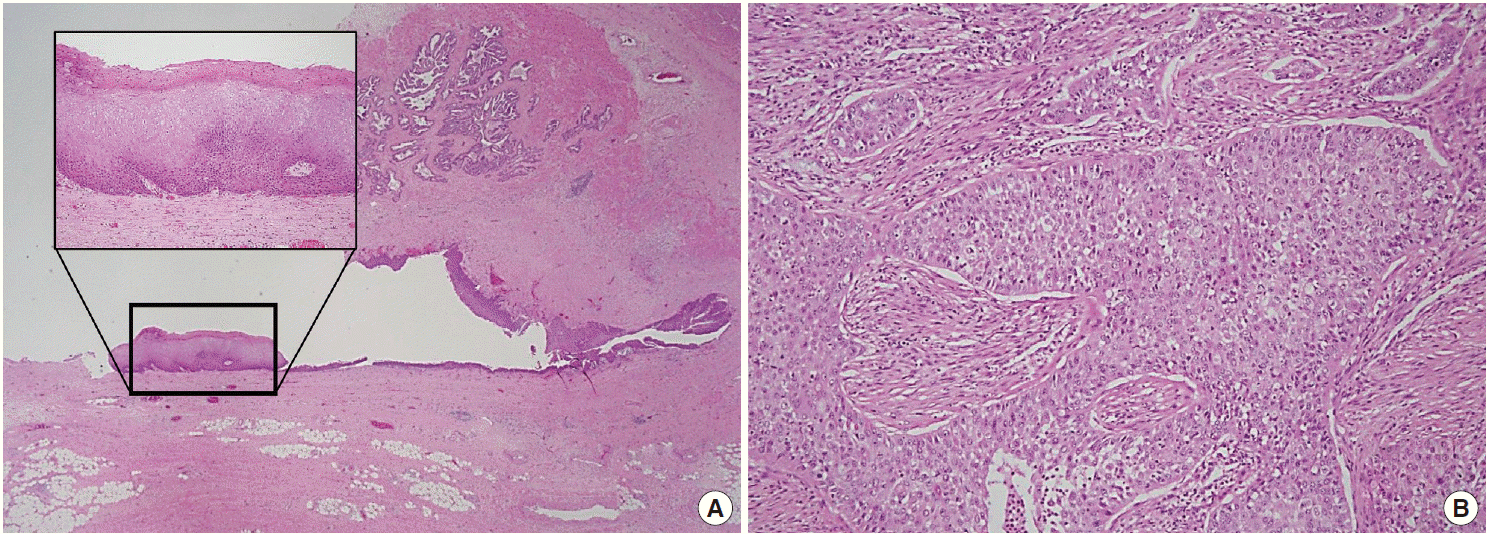
Fig. 3.
Immunohistochemistry and histochemical staining for phenotyping of the carcinoma. Tumor cells are positive for p63 (A), and negative for cytokeratin 7 (B), cytokeratin 20 (C), and carcinoembryonic antigen (D). Vimentin shows focal positivity in macrophages (E), and periodic acid–Schiff staining is positive for mucin (F).
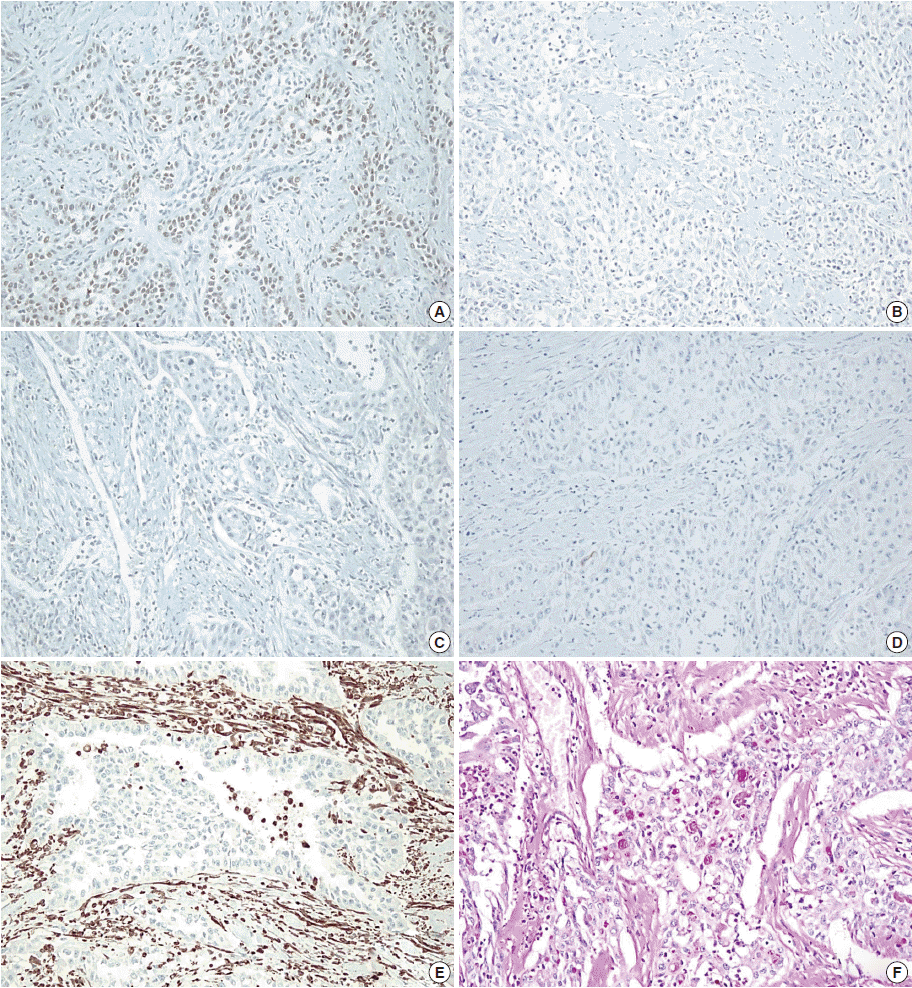
Table 1.
Summary of reported cases of carcinomas arising from a seminal vesicle cyst in Zinner syndrome patients
| Okada et al. (1992) [8] | Lee et al. (2007) [9] | Present case | |
|---|---|---|---|
| Age (yr) | 17 | 41 | 41 |
| Chief complaint | Lower abdominal mass and dysuria | Hematuria | Hematuria |
| Past medical history | None | Prostatitis | Squamous metaplasia, stone formation and chronic inflammation of the seminal vesicle |
| Seminal vesicle cyst detection | Latest admission | Latest admission | Twelve years ago |
| Location | Right seminal vesicle cyst | Left seminal vesicle cyst | Right seminal vesicle cyst |
| Pathologic diagnosis | Papillary adenocarcinoma | Mucinous adenocarcinoma | Poorly differentiated squamous cell carcinoma |
| Prostate-specifi antigen | Normal | Normal | Normal |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download