This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Polo-like kinase 4 (PLK4) is a serine/threonine protein kinase located in the centriole of the chromosome during the cell cycle. PLK4 overexpression has been described in a variety of many common human epithelial tumors. Conversely, PLK4 acts as a haploinsufficient tumor suppressor in some situations, highlighting the importance of strict regulation of PLK4 expression, activity, and function. Meanwhile, the importance of chemoradiation resistance in rectal cancer is being emphasized more than ever. We aimed to analyze PLK4 expression and the tumor regression grade (TRG) in patients with rectal cancer, treated with chemoradiotherapy (CRT).
Methods
A retrospective study was conducted on 102 patients with rectal cancer who received preoperative CRT. Immunohistochemistry for PLK4 in paraffin-embedded tissue was performed from the biopsy and surgical specimens.
Results
We found significant association between high expression of PLK4 and poor response to neoadjuvant CRT (according to both Mandard and The Korean Society of Pathologists TRG systems) in the pre-CRT specimens. Other clinicopathologic parameters did not reveal any correlation with PLK4 expression.
Conclusions
This study revealed an association between high expression of PLK4 in the pre-CRT specimens and TRG. Our results indicated that PLK4 could potentially be a new predictor for CRT effect in patients with rectal cancer.
Keywords: Polo-like kinase 4, Rectal neoplasms, Chemoradiotherapy, Biomarker
The 5-year survival rate for rectal cancer patients undergoing surgery is 50%. This is because even with curative resection, the risk of local recurrence or distant metastasis is high [
1]. Therefore, improving survival remains an important issue, even in resectable rectal cancer. Meanwhile, in patients with rectal cancer, preoperative chemoradiotherapy (CRT) significantly improves the overall and cancer-specific survival rates compared to surgery alone. Therefore, chemoradioresistance is an important challenge in rectal cancer and a major aspect of its treatment.
Polo-like kinase 4 (PLK4) is a serine/threonine kinase located in the centriole of the chromosome throughout the cell cycle and is essential for centriole duplication [
2–
5]. PLK4 expression has been described in a variety of human solid tumors [
6]. In particular, high levels of PLK4 mRNA have been detected in triple-negative breast cancers, which are resistant to conventional systemic therapy [
7]. PLK4 expression upregulation independently induces aneuploidy, loss of cell polarity, and hyperplasia in certain non-transformed cell lines and proliferative tissues [
8,
9]. In the context of p53 dysfunction, elevated PLK4 expression contributes to aneuploidy and tumorigenesis [
9,
10]. Contrarily, PLK4 acts as a haplo-insufficient tumor suppressor in some situations. Rosario et al. pointed out that haploid levels of PLK4 interfered with RhoGTPase function during cytokinesis, leading to aneuploidy and tumorigenesis, thus suggesting that early loss of heterozygosity in PLK4 is one of the factors leading to hepatocellular carcinogenesis [
11,
12]. These results underscore the importance of strict regulation of PLK4 expression, activity, and function [
13]. However, studies on PLK4 expression in patients with rectal cancer are limited. Thus, this study aimed to retrospectively analyze PLK4 expression in patients with rectal carcinoma treated with preoperative CRT in both preoperative biopsy specimens and postoperative surgical specimens.
MATERIALS AND METHODS
Patients and sample collection
The electronic records of rectal cancer patients treated at Keimyung University Dongsan Hospital were reviewed retrospectively. Among them, 102 cases containing both biopsy and surgical specimens were chosen. These tissue specimens along with patient clinical data (age, sex, date of surgery, recurrence, death, data on time to recurrence or death) and records of clinicopathological features (differentiation, lymphovascular invasion, pT category, pN category, pM category, microsatellite instability [MSI] status, KRAS, NRAS and BRAF mutations) of the rectal cancer were collected.
Among the various grading systems devised to evaluate chemoradioresistance, the Mandard system [
14] was used (Mandard-TRG [tumor regression grade]). Additionally, the KSP-TRG system, newly proposed by the Gastrointestinal Pathology Research Group of the Korean Society of Pathologists (KSP), based on the 8th edition of American Joint Committee on Cancer and the College of American Pathologists cancer protocol, was also used and data collected [
15]. This grade is divided into four tiers: grade 0 (no viable cancer cells, complete response), grade 1 (single cells or rare small groups of cancer cells, near-complete response), grade 2 (residual cancer with evident tumor regression, but more than single cells or rare small groups of cancer cells, partial response), grade 3 (extensive residual cancer with no evident tumor regression, poor or no response).
Overall survival (OS) was defined as the duration from the date of surgery to the date of the last follow-up visit or the date of death due to any cause, whereas disease-free survival (DFS) was defined as the duration from surgery to any type of recurrence.
Immunohistochemistry
We selected formalin-fixed paraffin-embedded (FFPE) tissue samples from rectal cancer tissues and used them for tissue microarray (TMA) construction. Of the 102 patients with rectal cancer, tissue specimens from both before CRT (biopsy specimen) and after CRT (surgery specimen) were selected for 71 patients. Only biopsy specimens were selected from 31 patients with no or few cells remaining in the surgical specimens. Representative tumor areas were identified by two pathologists (H.O. and H.W.L.) in hematoxylin and eosin-stained tissue sections. After the pathologists’ review, TMAs were assembled from triplicate 5 mm cores of the FFPE tumor samples. Immunohistochemical staining was performed on the TMA sections with an antibody against PLK4 (12952-1-AP, 1:400, ProteinTech Group, Inc., Chicago, IL, USA) using the Ventana BenchMark XT Automated System following the manufacturer’s protocol (Ventana Medical Systems, Tucson, AZ, USA).
Evaluation of immunohistochemistry
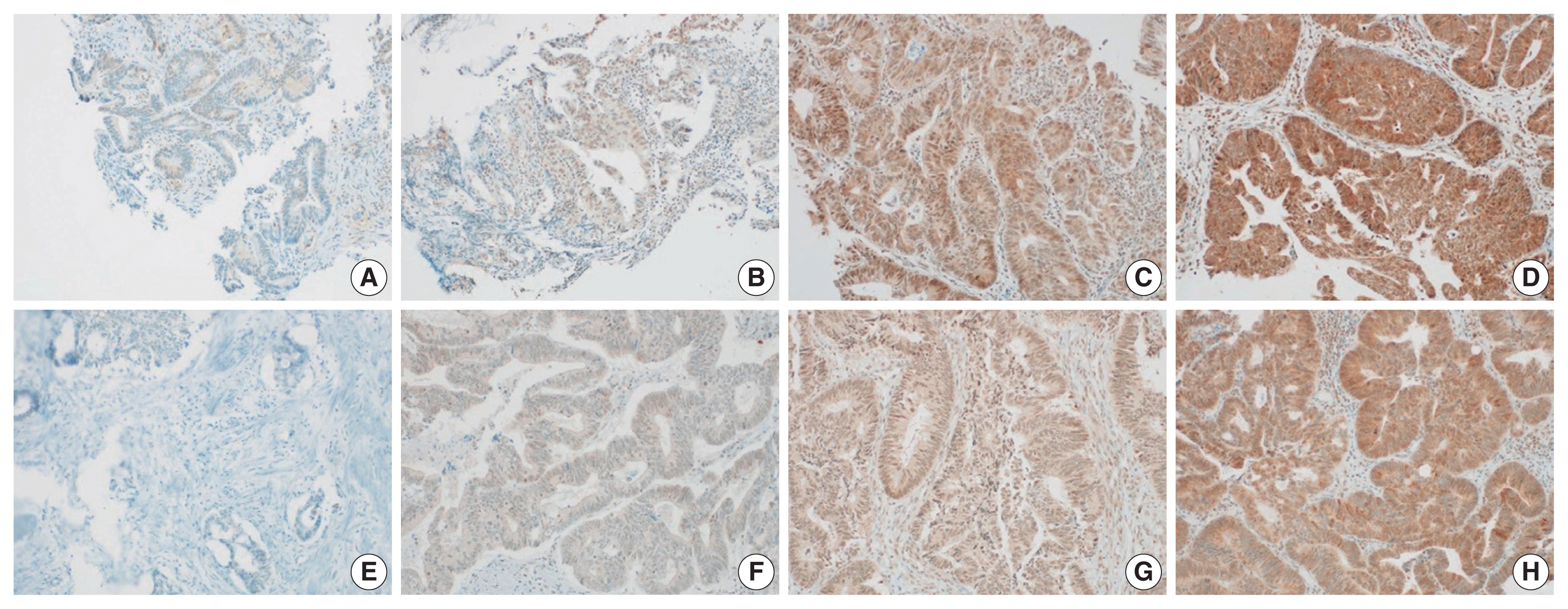
The histoscore (H-score) method was used to determine the immunohistochemical expression of PLK4. The H-score was calculated by multiplying the intensity score by the percentage of positive cells and ranges from 0 to 300 points [
16]. We scored both the nucleus and cytoplasm of previously selected samples. The intensities were scored as “0” (negative staining), “1” (weak staining), “2” (medium staining), and “3” (strong staining) (
Fig. 1). Based on the median H-score, two groups were formed for statistical analysis: “high expression” (H-score > median) and “low expression” (H-score ≤ median).
Fig. 1
Immunohistochemistry analyses of PLK4 expression in rectal cancer tissues. The expression of PLK is evaluated by H-score; intensity multiplied by percentage of positive cells. (A) Score 0 (negative staining) of PLK4 expression of pre-CRT specimen. (B) Score 1 (weak staining) of PLK4 expression of pre-CRT specimen. (C) Score 2 (medium staining) of PLK4 expression of pre-CRT specimen. (D) Score 3 (strong staining) of PLK4 expression of pre-CRT specimen. (E) Score 0 (negative staining) of PLK4 expression of post-CRT specimen. (F) Score 1 (weak staining) of PLK4 expression of post-CRT specimen. (G) Score 2 (medium staining) of PLK4 expression of post-CRT specimen. (H) Score 3 (strong staining) of PLK4 expression of post-CRT specimen. PLK4, Polo-like kinase 4; CRT, chemoradiotherapy.

Statistical analysis
All analyses were performed using IBM SPSS Statistics software ver. 26.0 (IBM Corp., Armonk, NY, USA). Chi-square with Fischer’s exact tests analysis were performed. Kaplan-Meier curves were used for survival analysis. Ordinal logistic regression model was used to compare the influence of individual factors, including age, sex, pT category, pN category, MSI status, and nuclear and cytoplasmic PLK4 expression of the pre-CRT specimen, on the KSP-TRG system. A two-tailed p-value of less than .05 indicated statistical significance.
RESULTS
Cohort characteristics
The median age of the cohort was 65 years (range, 32 to 85 years). Among the 102 patients, 70 (69%) were men and 32 (31%) were women. Rectal cancer recurred in 28 patients (27%), and 11 patients (11%) died. All characteristics of the 102 patients with rectal cancer are shown in
Table 1.
Table 1
Clinicopathological parameters in 102 cases of rectal cancer patients
|
Characteristic |
No. (%) |
|
Age (yr) |
|
< 65 |
50 (49.0) |
|
≥ 65 |
52 (51.0) |
|
Median (range) |
65 (32–85) |
|
Sex |
|
Male |
70 (68.6) |
|
Female |
32 (31.4) |
|
Differentiation |
|
Well |
5 (4.9) |
|
Moderate |
89 (87.3) |
|
Poorly |
5 (4.9) |
|
Lymphovascular invasion |
|
Absent |
71 (69.6) |
|
Present |
23 (22.5) |
|
pT category |
|
Tx–T2 |
50 (49.0) |
|
T3–T4 |
52 (51.0) |
|
pN category |
|
N0 |
71 (69.6) |
|
N1–N2 |
31 (30.4) |
|
pM category |
|
M0 |
101 (99.0) |
|
M1 |
1 (1.0) |
|
TRG of Mandard (group) |
|
TRG1, 2 |
27 (26.5) |
|
TRG3 |
37 (36.3) |
|
TRG4, 5 |
38 (37.3) |
|
TRG of KSP (group) |
|
TRG0, 1 |
27 (26.5) |
|
TRG2, 3 |
75 (73.5) |
|
TRG of Mandard |
|
TRG1 |
16 (15.7) |
|
TRG2 |
11 (10.8) |
|
TRG3 |
37 (36.3) |
|
TRG4 |
28 (27.5) |
|
TRG5 |
10 (9.8) |
|
TRG of KSP |
|
TRG0 |
16 (15.7) |
|
TRG1 |
11 (10.8) |
|
TRG2 |
39 (38.2) |
|
TRG3 |
36 (35.3) |
|
MSI |
|
MSS and MSI-L |
93 (91.2) |
|
MSI-H |
9 (8.8) |
|
Recurrence |
|
No |
74 (72.5) |
|
Yes |
28 (27.5) |
|
Death |
|
No |
91 (89.2) |
|
Yes |
11 (10.8) |

PLK4 expression and TRG
High nuclear PLK4 expression in pre-CRT specimens (H-score > 160) correlated with high Mandard-TRG (p < .001) and KSP-TRG (p < .001). The Mandard-TRG (p < .001) and KSP-TRG (p < .001) also showed a significant correlation in the group. Furthermore, high cytoplasmic PLK4 expression in the pre-CRT specimens (H-score > 155) correlated with high Mandard-TRG (p = .022) and KSP (p = .013) systems. Again, the Mandard-TRG (p = .002) and KSP-TRG (p = .004) were also significantly correlated within groups. There was no association between PLK4 expression in the pre-CRT specimens and other parameters. The results mentioned above are summarized in
Table 2. Nuclear PLK4 expression in the post-CRT specimens was correlated with sex (p = .015) and mortality (p = .035). There was no association between PLK4 expression in the post-CRT specimens and other parameters. The results mentioned above are summarized in
Table 3.
Table 2
Correlation of PLK4 expression of pre-CRT specimen with clinicopathological parameters in 102 cases of rectal cancer patients
|
Nuclear stain of PLK4 in pre-CRT specimen |
Cytoplasmic stain of PLK4 in pre-CRT specimen |
|
|
|
Low expression (H-score ≤ 160) |
High expression (H-score > 160) |
p-value |
Low expression (H-score ≤ 155) |
High expression (H-score > 155) |
p-value |
|
Age (yr) |
|
|
.196 |
|
|
> .99 |
|
< 65 |
18 (20.9) |
24 (27.9) |
|
21 (24.4) |
21 (24.4) |
|
|
≥ 65 |
25 (29.1) |
19 (22.1) |
|
22 (25.6) |
22 (25.6) |
|
|
Sex |
|
|
.486 |
|
|
.816 |
|
Male |
31 (36.0) |
28 (32.6) |
|
29 (33.7) |
30 (34.9) |
|
|
Female |
12 (14.0) |
15 (17.4) |
|
14 (16.3) |
13 (15.1) |
|
|
Differentiation |
|
|
.999 |
|
|
.999 |
|
Well |
2 (2.4) |
2 (2.4) |
|
2 (2.4) |
2 (2.4) |
|
|
Moderate |
39 (45.9) |
38 (44.7) |
|
39 (45.9) |
38 (44.7) |
|
|
Poorly |
2 (2.4) |
2 (2.4) |
|
2 (2.4) |
2 (2.4) |
|
|
Lymphovascular invasion |
|
|
.118 |
|
|
.150 |
|
Absent |
26 (32.5) |
35 (43.8) |
|
27 (33.8) |
34 (42.5) |
|
|
Present |
12 (15.0) |
7 (8.8) |
|
12 (15.0) |
7 (8.8) |
|
|
pT category |
|
|
.518 |
|
|
.518 |
|
Tx–T2 |
23 (26.7) |
20 (23.3) |
|
20 (23.3) |
23 (26.7) |
|
|
T3–T4 |
20 (23.3) |
23 (26.7) |
|
23 (26.7) |
20 (23.3) |
|
|
pN category |
|
|
.323 |
|
|
.138 |
|
N0 |
30 (34.9) |
34 (39.5) |
|
29 (33.7) |
35 (40.7) |
|
|
N1–N2 |
13 (15.1) |
9 (10.5) |
|
14 (16.3) |
8 (9.3) |
|
|
pM category |
|
|
> .99 |
|
|
.309 |
|
M0 |
42 (49.4) |
42 (49.4) |
|
41 (48.2) |
43 (50.5) |
|
|
M1 |
1 (1.2) |
0 |
|
1 (1.2) |
0 |
|
|
TRG of Mandard |
|
|
< .001 |
|
|
.022 |
|
TRG1, 2 |
19 (22.1) |
3 (3.5) |
|
16 (18.6) |
6 (7.0) |
|
|
TRG3 |
13 (15.1) |
19 (22.1) |
|
16 (18.6) |
16 (18.6) |
|
|
TRG4, 5 |
11 (12.8) |
21 (24.4) |
|
11 (12.8) |
21 (24.4) |
|
|
TRG of KSP |
|
|
< .001 |
|
|
.013 |
|
TRG0, 1 |
19 (22.1) |
3 (3.5) |
|
16 (18.6) |
6 (7.0) |
|
|
TRG2, 3 |
24 (27.9) |
40 (46.5) |
|
27 (31.4) |
37 (43.0) |
|
|
MSI |
|
|
> .99 |
|
|
> .99 |
|
MSS and MSI-L |
39 (45.3) |
38 (44.2) |
|
37 (52.1) |
28 (39.4) |
|
|
MSI-H |
4 (4.7) |
5 (5.8) |
|
4 (5.6) |
2 (2.8) |
|
|
Recurrence |
|
|
.952 |
|
|
.357 |
|
No |
32 (37.6) |
33 (33.8) |
|
31 (44.3) |
19 (27.1) |
|
|
Yes |
10 (23.8) |
10 (11.8) |
|
10 (14.3) |
10 (14.3) |
|
|
Death |
|
|
> .99 |
|
|
.724 |
|
No |
40 (46.5) |
39 (45.3) |
|
35 (49.3) |
27 (38.0) |
|
|
Yes |
3 (3.5) |
4 (4.7) |
|
6 (8.5) |
3 (4.2) |
|

Table 3
Correlation of PLK4 expression of post-CRT specimen with clinicopathological parameters in 102 cases of rectal cancer patients
|
Nuclear stain of PLK4 in post-CRT specimen |
Cytoplasmic stain of PLK4 in post-CRT specimen |
|
|
|
Low expression (H-score ≤160) |
High expression (H-score >160) |
p-value |
Low expression (H-score ≤155) |
High expression (H-score >155) |
p-value |
|
Age (yr) |
|
|
.252 |
|
|
.897 |
|
< 65 |
17 (25.4) |
18 (26.9) |
|
28 (41.8) |
7 (10.4) |
|
|
≥ 65 |
20 (29.9) |
12 (17.9) |
|
26 (38.8) |
6 (9.0) |
|
|
Sex |
|
|
.015 |
|
|
.526 |
|
Male |
30 (44.8) |
16 (23.9) |
|
38 (56.7) |
8 (11.9) |
|
|
Female |
7 (10.4) |
14 (20.9) |
|
16 (23.9) |
5 (7.5) |
|
|
Differentiation |
|
|
.251 |
|
|
.332 |
|
Well |
0 |
2 (3.1) |
|
1 (1.6) |
1 (1.6) |
|
|
Moderate |
34 (53.1) |
24 (37.5) |
|
47 (73.4) |
11 (17.2) |
|
|
Poorly |
2 (3.1) |
2 (3.1) |
|
4 (6.3) |
0 |
|
|
Lymphovascular invasion |
|
|
.849 |
|
|
.318 |
|
Absent |
26 (39.4) |
21 (31.8) |
|
36 (54.5) |
11 (16.7) |
|
|
Present |
11 (16.7) |
8 (12.1) |
|
17 (25.8) |
2 (3.0) |
|
|
pT category |
|
|
.057 |
|
|
.317 |
|
Tx–T2 |
8 (11.9) |
13 (19.4) |
|
15 (22.4) |
6 (9.0) |
|
|
T3–T4 |
29 (43.3) |
17 (25.4) |
|
39 (58.2) |
7 (10.4) |
|
|
pN category |
|
|
.154 |
|
|
.753 |
|
N0 |
26 (38.8) |
16 (23.9) |
|
33 (49.3) |
9 (13.4) |
|
|
N1–N2 |
11 (16.4) |
14 (20.9) |
|
21 (31.3) |
4 (6.0) |
|
|
pM category |
|
|
> .99 |
|
|
.197 |
|
M0 |
36 (54.5) |
29 (43.9) |
|
53 (80.3) |
12 (18.2) |
|
|
M1 |
1 (1.5) |
0 |
|
0 |
1 (1.5) |
|
|
TRG of Mandard |
|
|
.918 |
|
|
.279 |
|
TRG1, 2 |
2 (3.0) |
1 (1.5) |
|
3 (4.5) |
0 |
|
|
TRG3 |
16 (23.9) |
13 (19.4) |
|
21 (31.3) |
8 (11.9) |
|
|
TRG4, 5 |
19 (28.4) |
16 (23.9) |
|
30 (44.8) |
5 (7.5) |
|
|
TRG of KSP |
|
|
> .99 |
|
|
> .99 |
|
TRG0, 1 |
2 (3.0) |
1 (1.5) |
|
3 (4.5) |
0 |
|
|
TRG2, 3 |
35 (52.2) |
29 (43.3) |
|
51 (76.1) |
13 (19.4) |
|
|
MSI |
|
|
> .99 |
|
|
.574 |
|
MSS and MSI-L |
34 (50.7) |
28 (41.8) |
|
49 (73.1) |
13 (19.4) |
|
|
MSI-H |
3 (4.5) |
2 (3.0) |
|
5 (7.5) |
0 |
|
|
Recurrence |
|
|
.671 |
|
|
.314 |
|
No |
25 (37.9) |
21 (31.8) |
|
35 (53.0) |
11 (16.7) |
|
|
Yes |
12 (18.2) |
8 (12.1) |
|
18 (27.3) |
2 (3.0) |
|
|
Death |
|
|
.035 |
|
|
.677 |
|
No |
29 (43.3) |
29 (43.3) |
|
46 (68.7) |
12 (17.9) |
|
|
Yes |
8 (11.9) |
1 (1.5) |
|
8 (11.9) |
1 (1.5) |
|

High Mandard-TRG correlated with high pT category (p < .001), high pN category (p = .026), microsatellite instability–high (p = .049), and high KSP-TRG (p < .001). There was no association between the Mandard-TRG and other parameters. The results mentioned above are summarized in
Supplementary Table S1. High KSP-TRG correlated with high pT category (p < .001), high pN category (p = .011), and high Mandard-TRG (p < .001). There was no association between the KSP-TRG and other parameters. The results mentioned above are summarized in
Supplementary Table S2.
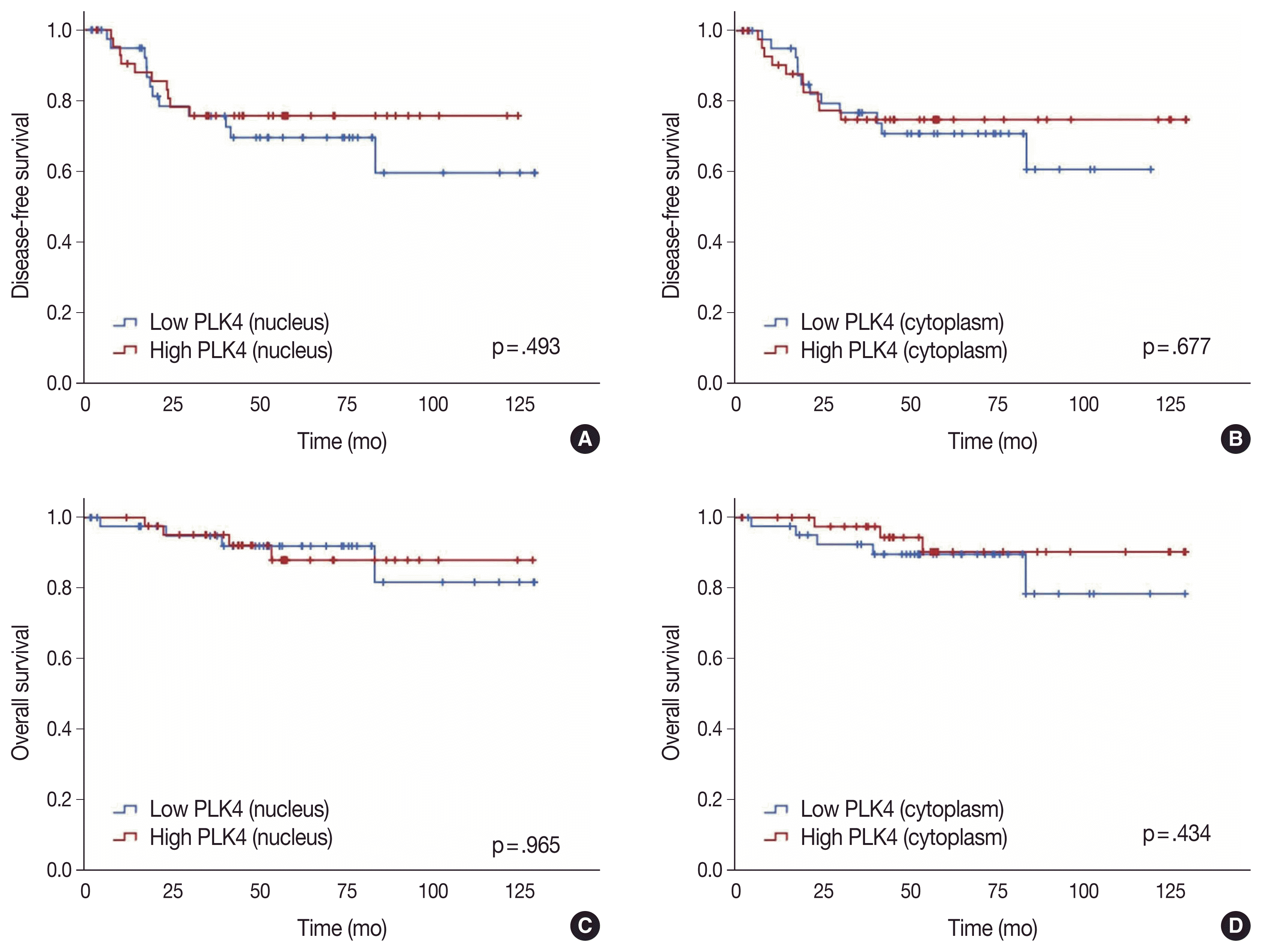
Conversely, there was no significant association between DFS and high nuclear expression of PLK4 (p = .493) (
Fig. 2A) or high cytoplasmic expression of PLK4 (p = .677) (
Fig. 2B) in the pre-CRT specimens. There was also no significant association between OS and high nuclear expression of PLK4 (p = .965) (
Fig. 2C) or high cytoplasmic expression of PLK4 (p = .434) (
Fig. 2D) in the pre-CRT specimens.
Fig. 2
(A) Kaplan-Meier curves of disease-free survival in high and low expression of nuclear expression of PLK4 of pre-CRT specimen. (B) Kaplan-Meier curves of disease-free survival in high and low expression of cytoplasmic expression of PLK4 of pre-CRT specimen. (C) Kaplan-Meier curves of overall survival in high and low nuclear expression of PLK4 of pre-CRT specimen. (D) Kaplan-Meier curves of overall survival in high and low expression of cytoplasmic expression of PLK4 of pre-CRT specimen. PLK4, Polo-like kinase 4; CRT, chemoradiotherapy.

Multivariate analysis for the KSP-TRG
Multivariate analysis showed that pT category and nuclear and cytoplasmic PLK4 expression in the pre-CRT specimens were statistically associated with the KSP-TRG (p < .05). Other variables, including age, sex, pN category, and MSI status, were not associated with the KSP-TRG. In addition, pT category and nuclear and cytoplasmic PLK4 expression in the pre-CRT specimens were statistically independent of each other or other variables. The results mentioned above are summarized in
Table 4.
Table 4
Multivariate analysis (ordinal logistic regression test) of TRG of KSP associated with clinicopathologic variables including nuclear and cytoplasmic PLK4 stain of pre-CRT specimen
|
Variable |
Estimate |
p-value |
95% Confidence interval |
|
Including nuclear PLK4 stain of pre-CRT specimen |
|
Age |
−0.685 |
.113 |
−1.532 to 0.161 |
|
Sex |
−0.173 |
.708 |
−1.080 to 0.734 |
|
pT category |
−1.403 |
.003 |
−2.338 to −0.469 |
|
pN category |
−0.520 |
.330 |
−1.567 to 0.527 |
|
MSI state |
−0.578 |
.406 |
−1.939 to 0.784 |
|
Nuclear PLK4 stain of pre-CRT specimen |
−1.766 |
< .001 |
−2.667 to −0.866 |
|
Including cytoplasmic PLK4 stain of pre-CRT specimen |
|
Age |
−0.411 |
.329 |
−1.237 to 0.415 |
|
Sex |
−0.351 |
.444 |
−1.250 to 0.548 |
|
pT category |
−1.595 |
.001 |
−2.532 to −0.658 |
|
pN category |
−0.513 |
.335 |
−1.558 to 0.531 |
|
MSI state |
−0.444 |
.530 |
−1.829 to 0.940 |
|
Cytoplasmic PLK4 stain of pre-CRT specimen |
−1.500 |
.001 |
−2.376 to −0.623 |

DISCUSSION
In this study, we first distinguished the expression of immunohistochemical staining of PLK4 in samples before and after CRT by its high and low expressions and classified them according to the clinical data, including the prognosis and histopathological features. We also found that the expression of PLK4 in the pre-CRT specimens was associated with both the Mandard-TRG and KSP-TRG. This association was found in both nuclear and cytoplasmic expression. Therefore, preoperative evaluation of PLK4 will be useful as one of the methods to predict the response of rectal cancer patients to CRT. Unfortunately, the association between PLK4 and prognosis was not revealed in this study and more robust studies are needed in the future to support this possibility.
Although radiation therapy before surgery reduces the local recurrence rate by more than 50% than surgery alone [
1] and treatment for rectal cancer has greatly evolved in recent years, patients are prone to recurrence and many eventually die from the disease. Therefore, the importance of the response to preoperative CRT in rectal cancer is very important.
In a study on the correlation between preoperative CRT and PLK4 expression, PLK4 enhanced chemoradiation resistance in glioblastoma multiforme (GBM), whereas PLK4 knockdown via lentiviral transfection significantly increased the chemoradiation sensitivity of GBM cells [
17]. The authors stated that PLK4 was transcriptionally regulated depending on
ATAD2 (ATPase family AAA domain containing 2), a gene involved in cell proliferation and metastasis, and that this regulation increased PLK4 radioresistance. In addition, PLK4 expression is known to be a negative predictor of response to taxane-based neoadjuvant chemotherapy [
18]. The authors stated that the resistance to taxane-based chemotherapy appeared in patients with high PLK4 expression because PLK4 was involved as an upstream regulator of gamma-tubulin, which affected the treatment response to taxane. Therefore, the authors concluded that PLK4 inhibitors enhanced the effect of chemotherapy. However, reports on the possibility of prognostic effects of PLK4 expression in rectal cancer are lacking.
There are several drawbacks to our study. The most significant drawback was the limited number of cases. Despite the small sample size that could undermine the power of the study, we found a significant difference, which should be confirmed by large-scale studies in the future. Additionally, the retrospective nature of the study was a limitation to its design. It would also be necessary to design a better-validated study with no missing factors. Future research with better power should be conducted to confirm the preliminary results obtained from this study.
We reported PLK4 expression and the prognosis of rectal cancer by dividing our study into pre-CRT and post-CRT groups. Based on our human tissue study results, PLK4 expression could be a potential predictor for the response of CRT. Therefore, the clinical development of PLK4 could be an effective therapeutic strategy for the management of advanced rectal cancer.



