This article has been
cited by other articles in ScienceCentral.
Abstract
SMARCA4/BRG1-deficient lung adenocarcinoma (SD-LUAD) is being recognized as a distinct subtype based on subtle differences in its clinical, morphological, and immunophenotypic attributes compared to other non–small cell lung carcinomas. We present here a case of SD-LUAD with curious thyroid transcription factor 1 (TTF1) expression in a morphologically heterogenous lung adenocarcinoma. The better differentiated area showed preservation of TTF1 expression, and a poorly differentiated tumor had loss of TTF1 expression with universal BRG1 loss.
Go to :

Keywords: BRG1, Lung, Non-small cell lung carcinoma, SMARCA4, Thyroid transcription factor 1
Mammalian switch/sucrose non-fermentable (SWI/SNF) complex is a major chromatin remodeling pathway comprising of three orthologues. Of these, BRG1/BRM-associated factor (BAF) is most significant with
SMARCA4 and
SMARCA2 providing energy through their ATPase like enzymatic activity [
1].
SMARCA4 gene encodes BRG1 (Brahma related gene), which is a catalytic subunit of the SWI/SNF complex. Several
SMARCA4 mutated cancers have been described, of these small cell carcinomas of ovary, hypercalcemic-type, and thoracic tumors have been best studied. It is widely believed that
SMARCA4/BRG1-deficient lung adenocarcinoma (SD-LUAD) is devoid of thyroid transcription factor 1 (TTF1) expression though a contrary view exists [
2–
5]. We report a case of morphologically heterogenous lung adenocarcinoma with heterogenous TTF1 expression and universal BRG1 loss.
CASE REPORT
A 54-year-old male, chronic smoker with a 40 pack-year history, presented with complaints of breathlessness and hemoptysis. Routine laboratory investigations were within normal limits. Chest X-ray revealed a large rounded mass in the parahilar region of the left lung with mediastinal widening. Whole-body positron emission tomography and non-diagnostic computed tomography (PET-CT) revealed a parahilar mass in the left lung measuring 6.2 × 4.8 cm with bulky mediastinal lymphadenopathy and bony metastases (
Fig. 1). Computed tomography (CT)–guided trucut biopsy from the left lung mass revealed a heterogeneous tumor with solid pattern (
Fig. 2A). The tumor cells in the better differentiated area had bland nuclei, high nuclear to cytoplasmic ratio, and a compact morphology (
Fig. 2B, C). The tumor cells in other areas had abundant pink bubbly cytoplasm, pleomorphic vesicular nuclei with prominent nucleoli, and brisk mitoses (
Fig. 2D, E). Neutrophilic emperipolesis and inflamed stroma were also seen in these areas (
Fig. 2D, E).
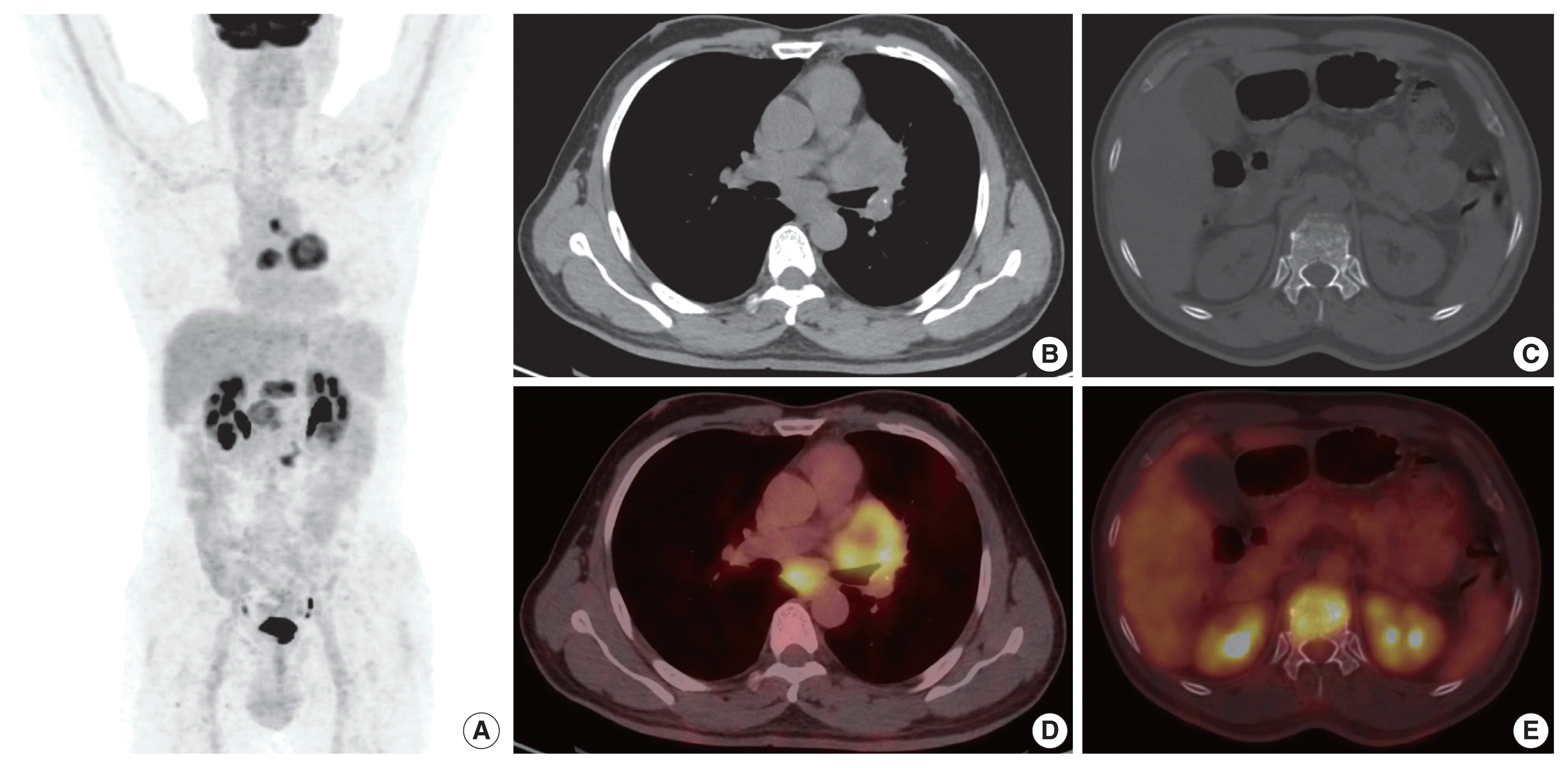 | Fig. 1Positron emission tomography computed tomography (PET-CT) image. (A) Maximum intensity projection. (B, C) Axial computed tomography. (D, E) Fused fluorodeoxyglucose PET-CT axial images showing left lung central lesion with mediastinal lymph nodes and bone lesions. 
|
 | Fig. 2Histopathological findings of the case. (A) Scanner view depicting two cores: one with compact morphology with a trabecular and abortive cribriform pattern (left) and another more loose and pink owing to abundant cytoplasm (right). (B) Low power view of better differentiated area (compact morphology) with tumor cells arranged in acinar and abortive cribriform pattern. (C) Tumor cells in a better differentiated area showing uniform tumor cells with scant eosinophilic cytoplasm and high nucleocytoplasmic ratio. (D) Low power view of lesser differentiated area (loose area) with tumor cells showing abundant bubbly cytoplasm, pleomorphic nuclei, and inflamed stroma. (E) Tumor cells of a lesser differentiated area showing abundant eosinophilic cytoplasm, vesicular pleomorphic nuclei, and neutrophilic emperipolesis with stroma rich in inflammatory cells. 
|
Upon immunohistochemistry (IHC) analysis, tumor cells in the better differentiated area (
Fig. 3A) expressed cytokeratin (CK) (
Fig. 3B), but lacked BRG1 expression (
Fig. 3C). These tumor cells were positive for TTF1 (
Fig. 3D) while tumor cells in lesser differentiated area (
Fig. 3E) expressed weaker CK (
Fig. 3F), and were devoid of BRG1 (
Fig. 3G) and TTF1 (
Fig. 3H). In summary, tumor cells in both the areas were devoid of BRG1 and expressed CK, CK7, epithelial membrane antigen, and BerEp4, while TTF1 expression was noted only in better differentiated component. On additional IHC, the tumor cells in the better differentiated area revealed aberrant expression of SRY-box transcription factor (SOX) 2 and SOX11, and were negative for p40, Hep Par 1, spalt like transcription factor 4 (SALL4), and CD34 immunoexpression. Other areas with poor differentiation exhibited immunopositivity for SALL4 and were negative for p40, Hep Par 1, SOX2, SOX11, and CD34 expression. This contrasts with the well-defined expression of Hep Par 1 in SD-LUAD and SALL4, SOX2, and CD34 in
SMARCA4/BRG1-deficient thoracic sarcoma (SD-TS) [
4]. A final diagnosis of SD-LUAD was established on the basis of epithelial marker expression and BRG1 loss. Programmed death-ligand 1 immunoexpression showed a tumor proportion score of < 1%. No targetable driver mutation was identified for epidermal growth factor receptor (
EGFR), reactive oxygen species 1 (
ROS1), and anaplastic lymphoma kinase 1 (
ALK-1).
 | Fig. 3Representative images of immunohistochemistry. Tumor cells in a better differentiated area (A), showing cytokeratin (CK) expression in tumor cells (B), loss of BRG1 in tumor cells (C), thyroid transcription factor 1 (TTF1) expression by tumor cells in better differentiated area (D). Tumor cells in lesser differentiated area (E) showing weaker CK expression in tumor cells (F). Loss of BRG1 in tumor cells (G) and loss of TTF1 (H) in tumor cells showing poor differentiation. 
|
The patient received six cycles of 5 Gy radiotherapy, but unfortunately succumbed to his illness.
Go to :

DISCUSSION
The literature describes a varied morphology of SD-LUAD in the form of solid adenocarcinoma, large cell carcinoma, hepatoid, rhabdoid, spindling, and signet ring cell along with inflamed stroma, emperipolesis, necrosis, and brisk mitosis [
4–
9]. There is only a handful of cases with acinar/papillary morphology [
3,
6]. SD-LUAD are typically negative for TTF1 as described in the literature [
5,
6]. Contrary observations were described by Herpel et al. [
3] and Agaimy et al. [
4] with intact TTF1 expression in 20% and 10% of their cases, respectively. The present case is unique with heterogeneous tumor morphology and heterogenous TTF1 expression, but with diffuse loss of BRG1. Despite having a typical morphology of adenocarcinoma in one core, BRG1 immunoexpression was studied in view of the morphological telltale signs of SD-LUAD in other areas.
SD-LUAD can variably express Hep Par 1 and stem cell markers are more often expressed in SD-TS [
4]. Our case unfolds the dynamic and evolving genetic events of lung adenocarcinoma. TTF1 was expressed only by the better differentiated component and BRG1 loss was exhibited by both areas in the present case. The loss of BRG1 in the better differentiated component is germane to our hypothesis that BRG1 loss was the primary event, which eventually had downregulated the transcription of TTF1 and was reflected as loss of TTF1 in poorly differentiated area and suggested that
SMARCA4 mutation was the driver mutation. The observation of SALL4 expression in lesser differentiated area with aberrant SOX2 and SOX11 expression in better differentiated area is difficult to explain in the present case. However, it clearly unravels the evolutionary pathway of oncogenesis with SMARCA4 protein loss being the primary oncogenic event. There is continuous remodeling of chromatin responsible for gain and loss of many genes and protein expression, which is reflected as aberrant and heterogenous expression of SALL4, SOX2, and SOX11 in the present case. Rekhtman et al. also proposed that
SMARCA4-deficient thoracic sarcomas are indeed undifferentiated or dedifferentiated lung carcinoma associated with smoking [
10]. The IHC in the present case imitates the ongoing continuous remodeling, thereby leaving a probability of possible dedifferentiation of adenocarcinoma into sarcoma over time.
Given an almost indistinctive morphology, a need to minimize IHC, the unique biology propelling a rapid progression, and lack of actionable driver mutation, this entity needs separation from other lung adenocarcinoma, so that it can be studied more exhaustively to develop a genome-directed therapy. This task however, is rendered more difficult by the fact that some of the SD-LUAD may express TTF1, and this finding opens up the question whether all non–small cell lung carcinomas should be assessed for SMARCA4/BRG1 protein expression.
Go to :








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download