This article has been
cited by other articles in ScienceCentral.
Abstract
Testicular carcinoid tumors are very rare, accounting for less than 1% of all testicular tumors. We report a rare case of a testicular carcinoid tumor with extensive lymphatic invasion. A 42-year-old man presented with a painless, enlarged right testicular mass. There was no history of injury or discomfort in this region. Right radical orchiectomy was performed, which showed a well-defined, non-encapsulated solid white mass with calcification (7.0 × 4.5 × 3.5 cm) and absence of cystic components. Microscopic examination using hematoxylin and eosin staining of the tumor sections identified organoid, trabecular, and solid patterns with rosette formation. Extensive multifocal lymphatic invasion was observed. Immunohistochemistry was positive for synaptophysin, chromogranin, and CD56. Testicular carcinoid tumors usually show good prognoses; however, there was extensive lymphovascular invasion in this case. Thus, in the case of unusual presentation of the disease, close follow-up is necessary.
Go to :

Keywords: Testicular carcinoid tumor, Testicular neoplasm, Lymphovascular invasion, Orchiectomy
Testicular carcinoid tumors are very rare, with a prevalence of less than 1% among all testicular tumors [
1]. The first case of testicular carcinoid tumor was reported as an element of a benign cystic teratoma by Simon et al. in 1954 [
2]. Approximately 10% of testicular carcinoid tumors are complicated by the carcinoid syndrome [
3,
4]. Testicular carcinoid tumors can be divided into three subgroups: primary testicular carcinoid tumors, carcinoid tumors associated with teratoma, and carcinoid metastasis to the testis [
5].
Carcinoid tumors were initially considered benign [
6]; however, they are now regarded as potentially malignant neoplasms [
7]. Furthermore, benign and malignant carcinoid tumors are difficult to differentiate histologically, making it important to identify metastases. The preferred therapy for carcinoid tumors is surgical excision. Other therapies include somatostatin analogs, interferon-α, chemotherapeutics, and radiation. The treatment with somatostatin analogs is limited to symptom control in patients with carcinoid syndrome and stabilization of disease progression.
There are only a few studies on testicular carcinoid tumors, and the clinical implications of its diagnosis remain unclear. We report a very rare case of a carcinoid tumor of the testis with extensive lymphatic invasion but no manifestation of carcinoid syndrome.
CASE REPORT
A 42-year-old man presented with a painless, enlarged right testicular mass confirmed by ultrasonography (7.0 × 4.5 × 3.5 cm in size). The patient did not have a history of injury or discomfort in this region. A computed tomography scan revealed a 7.0 cm homogenous enhancing mass with calcification in the right testicle. Results of laboratory analysis were as follows: α-fetoprotein (AFP), 5.03 ng/mL (normal range, 0.1 to 930 ng/mL), beta-human chorionic gonadotropin, < 0.5 mIU/mL (normal range, 0 to 5 mIU/mL); and testosterone, 1.83 ng/mL (normal range, 1.2 to 10.19 ng/mL).
Right radical orchiectomy was performed, and it revealed a well-defined, non-encapsulated solid white mass with calcification (7.0 × 4.5 × 3.5 cm) and no cystic components. The cut surface was clear and evenly white, with no hemorrhage or necrosis (
Fig. 1). The spermatic cord did not show any abnormalities. Following the surgery, the patient has been alive and well for 13 months without lymph node metastasis or recurrence.
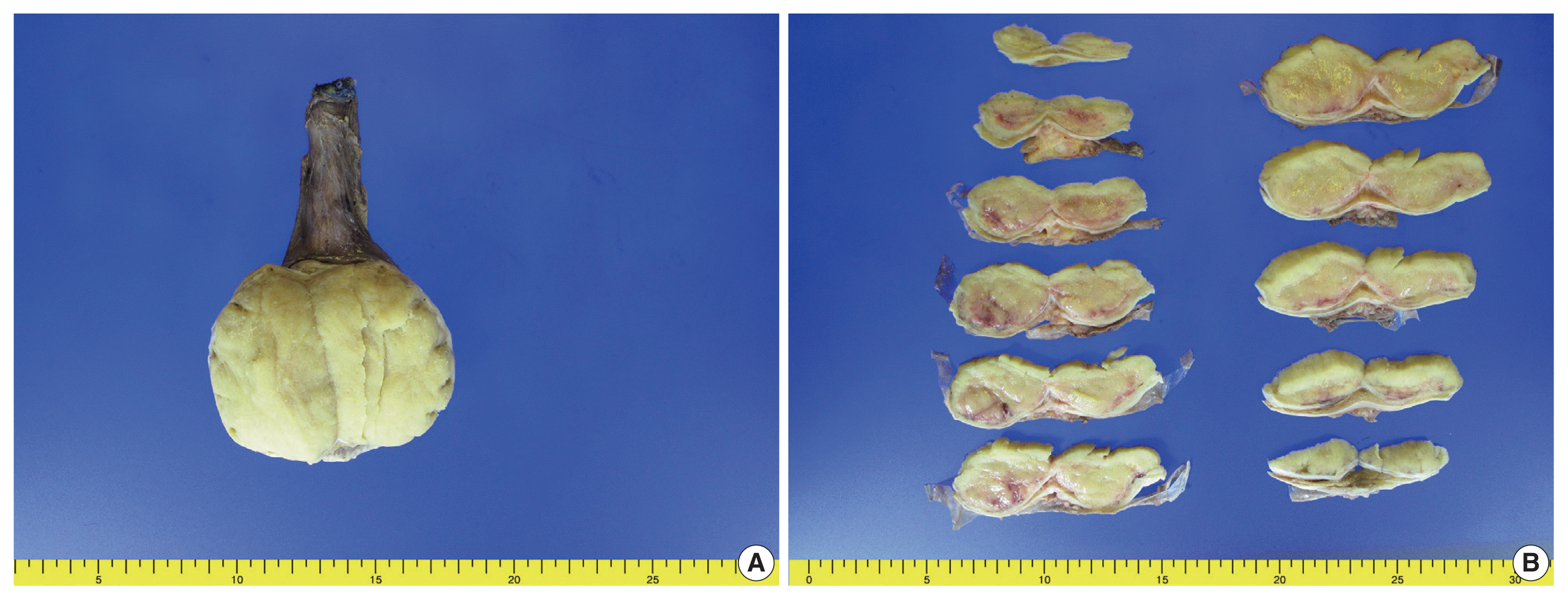 | Fig. 1Gross appearance of the right testicular mass. (A) A well-defined, non-encapsulated solid white mass is observed. (B) The cut surface is clear and evenly white without hemorrhage or necrosis. 
|
On microscopy, a hematoxylin and eosin stained tumor section showed organoid, trabecular, and solid patterns with rosette formation. The cells had a uniform polygonal shape with fine granular cytoplasm and round nuclei containing fine chromatin and small nucleoli (
Fig. 2A, B). The mitotic figure was 0 per high-power field (HPF). Ki67 proliferation index was less than 1%, and tumor necrosis was absent. Extensive multifocal lymphovascular invasion was identified (
Fig. 2C, D). There were no germ cell neoplasia in situ or other germ cell tumor components and no teratomatous component. The tumor had invaded the rete testis (pT2). Hemorrhage and necrosis were not observed. Further evaluation by immunohistochemistry showed the tumor was positive for synaptophysin, chromogranin, and CD56 (
Fig. 3A, B) and negative for GATA3, OCT3/4, CD30, AFP, and c-kit. CD31 showed positivity in the lymphovascular endothelium (
Fig. 3C), and D2-40 showed positivity in the lymphatic endothelium (
Fig. 3D). Based on the morphological and immunohistochemical findings, this case was diagnosed as primary testicular carcinoid tumor. The tumor was staged as pT2N0M0.
 | Fig. 2Microscopic findings of the testicular carcinoid tumor. (A) Organoid, trabecular, and solid patterns are visible. (B) The cells have a uniform polygonal appearance with fine granular cytoplasm and round nuclei containing fine chromatin and small nucleoli. (C, D) Extensive multifocal lymphatic invasion is detected. 
|
 | Fig. 3Immunohistochemical staining is positive for chromogranin and (A) synaptophysin (B). (C) CD31 shows positivity for lymphovascular endothelium. (D) D2-40 shows positivity for lymphatic endothelium. 
|
Go to :

DISCUSSION
The incidence of testicular tumors is low, and testicular carcinoid tumors account for only 1% of all testicular tumors [
8]. Primary testicular carcinoid tumors are exceedingly rare, and only a few cases have been reported in the literature. These tumors present in patients typically between their second and ninth decades of life, with an average diagnosis age of 46 years [
9,
10]. Testicular carcinoid tumors often do not elicit symptoms, or they cause symptoms similar to those of other testicular tumors. The symptoms are not specific, and the carcinoid syndromes are often not recognized until the histological diagnosis is confirmed.
In contrast to most ovarian carcinoid tumors that present as teratomatous components, most testicular carcinoid tumors are histologically pure [
11,
12]. Carcinoid tumors are 15 times more common in the ovaries than in the testicles. Ovarian carcinoid tumors are most often associated with mature cystic teratomas, which constitute the most common type of ovarian germ cell tumors in females up to 30 years of age [
13].
Histogenesis of testicular carcinoid tumors has not yet been defined. There is a strong rationale that testicular carcinoid tumors are of germ cell origin. Most tumor cases in the testes, especially solitary, large tumors, are likely to be primary. Testicular tumors are considered primary when metastasis from another primary tumor has been reasonably ruled out. Malignant outcomes have been reported in approximately 16% of cases. Features correlated with adverse outcomes include coagulative necrosis, 2 to 10 mitoses per 10 HPFs, atypical mitoses, and atypical carcinoid tumor-like features [
14]. The present case was primary testicular carcinoid tumor, and no metastasis was identified. Coagulative necrosis and mitosis, which are usually seen in atypical carcinoid tumor, were not identified. Compared to previously reported cases, this tumor showed extensive lymphovascular invasion without atypical carcinoid tumor pattern.
The key features of testicular carcinoid tumors are organoid and trabecular patterns, with tumor cells arranged in solid nests or cords. The tumor cells have uniform polygonal shapes with fine granular cytoplasm and round nuclei containing fine chromatin and small nucleoli.
Testicular carcinoid tumors usually have a good prognosis; however, when there is an unusual presentation of the disease, such as extensive lymphatic invasion as in this case, a close follow-up is necessitated. The presence of multifocal lymphovascular invasion in this case was striking and warranted close clinical follow-up and further work-up.
This is the first report of a primary testicular neuroendocrine tumor with extensive lymphatic invasion. Additionally, patients with malignant tumors exhibit a prolonged clinical course before death, which usually results from distant metastases. Therefore, a long-term follow-up evaluation is necessary for patients with this neoplasm.
Go to :






 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download