This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Radiation-induced organizing hematoma (RIOH) is a sporadic form of cavernous hemangioma (CH) that occurs after cerebral radiation. RIOH lesions are distinct histologically from de novo CH; however, detailed research on this subject is lacking. In the present study, the clinical and histological features of RIOHs were evaluated based on causative lesions.
Methods
The present study included 37 RIOHs confirmed by surgical excision from January 2009, to May 2020, in Yonsei Severance Hospital. All cases were divided into subgroups based on type of radiation treatment (gamma knife surgery [GKS], n = 24 vs. conventional radiation therapy [RT], n = 13) and pathology of the original lesion (arteriovenous malformation, n = 14; glioma, n = 12; metastasis, n = 4; other tumors, n = 7). The clinicopathological results were compared between the groups.
Results
Clinical data of multiplicity, latency, and size and wall thickness of the original tumors and RIOHs were analyzed. The GKS group showed shorter latency (5.85 ± 4.06 years vs. 11.15 ± 8.27 years, p = .046) and thicker tumor wall (693.7 ± 565.7 μm vs. 406.9 ± 519.7 μm, p = .049) than the conventional RT group. Significant difference was not found based on original pathology.
Conclusions
RIOH is more likely to occur earlier with thick tumor wall in subjects who underwent GKS than in patients who underwent conventional RT. These results indicate the clinical course of RIOH differs based on type of treatment and might help determine the duration of follow-up.
Keywords: Radiation-induced organizing hematoma, Cavernous hemangioma, Gamma knife surgery, Radiation therapy, Latency
Radiation-indicated cavernous hemangioma (RICH) refers to a localized vascular, tumor-like lesion that develops after cerebral radiation [
1–
3]. The lesion occurs mainly in children and young people, with variable latency periods after radiation treatment and diverse original lesions, including arteriovenous malformation (AVM), glioma, and metastatic tumor [
4,
5]. Similar to de novo cavernous hemangioma (CH), RICH appears as an enhancing lesion with popcorn-like appearance and partial hemosiderin rim on magnetic resonance imaging (MRI) and is observed histologically as a vascular-rich hemorrhagic lesion [
6–
8]. Thus, RICH has been regarded as a sporadic form of de novo CH that appears as a late complication of cerebral radiation [
9,
10].
Recently, in several studies in which RICH was compared with de novo CH, RICH was shown a potentially distinct group of diseases with different pathogenesis compared with previously known CHs [
6,
11–
13]. Previous research has reported that RICH following stereotactic radiosurgery shows some distinguishing features on MRI such as an unilocular cystic area with some solid component and prominent perilesional edema, and de novo CH appears to have a complete hemosiderin rim and less prominent perilesional edema [
6]. Furthermore, RICH is more likely to be an inactive organizing hematoma rather than a vascular malformation. Therefore, the term “radiation-induced organizing hematoma (RIOH)” was proposed to describe the lesions more appropriately and replace the term RICH, which might be a misnomer for these lesions [
6]. All the authors of the current study agreed to this concept, and the term RIOH, instead of RICH, was used throughout the manuscript.
Detailed studies on RIOH are limited. In particular, RIOH lesions have been reported irrespective of radiation dose, type of malignancy, or radiation type, such as gamma knife surgery (GKS) or conventional radiation therapy (RT) [
11]. However, studies to improve the understanding of RIOH lesions have not been conducted. In the present study, RIOH lesions were better defined, and their clinicopathological characteristics, including original pathology and type of radiation treatment, were compared.
MATERIALS AND METHODS
Patient selection and clinicopathological examination
Between January 2009 and May 2020, a total of 37 cases of pathologically confirmed RIOH was selected from Severance Hospital. For each case, the size of both the original tumor and RIOH was obtained. The size of original tumor was measured from preoperative imaging, and the size of RIOH were obtained through microscopic examination. The difference between the maximal diameter of primary tumor and RIOH was measured. All the pathologic slides were reviewed, and the tumor wall thickness of RIOH was measured at the thickest point of the submitted tissue under light microscope.
All medical records were reviewed, and clinical data including age at the time of RIOH detection, sex, radiologic findings, locations of the original tumor and RIOH, multiplicity, and latency period were compared between the original tumor and RIOH. A radiologist (M.P.) reviewed all MRI scans of patients, focusing on differences in radiologic findings including perilesional edema and hemosiderin rim depending on type of RT. Furthermore, information on previous treatments was collected. Cases were classified into two groups based on previous type of radiation (GKS, n = 24 vs. conventional RT, n = 13) and into four groups based on preceding pathology of the original tumor (AVM, n = 14; glioma, n = 12; metastasis, n = 4; other tumors, n = 7). The clinicopathological parameters were analyzed. For patients who had been treated multiple times or who underwent both GKS and RT, the groups and latency were divided and calculated based on timing and method of the last treatment.
Statistical analysis
Statistical analyses were performed using SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA). Continuous and categorical variables were analyzed using the non-parametric Mann-Whitney U test and the chi-square test, respectively, and p < .05 was considered statistically significant.
RESULTS
Patient characteristics
A total of 37 samples was included in the study. Baseline patient characteristics are summarized in
Table 1. RIOH samples were from 14 males (37.8%) and 23 females (62.2%). Twenty-four patients (64.9%) underwent GKS, and 13 (35.1%) were treated with conventional RT. Original tumor pathologies were as follows: AVM (n = 14, 37.8%), brain tumors (11 gliomas and 1 ependymoma; n = 12, 32.4%), metastasis (n = 4, 10.8%), and other tumors (3 schwannomas, two nasopharyngeal cancers, one pituitary tumor, and one craniopharyngioma; n = 7, 18.9%). The cases of nasopharyngeal cancer were subcategorized into ‘other tumors’ in this study because postoperative RT often is used for nasopharyngeal cancer, and the central nervous system area usually is included in the radiation field.
Clinicopathological differences between RIOH lesions
The overall results of the clinical and pathological comparisons are summarized in
Tables 2 and
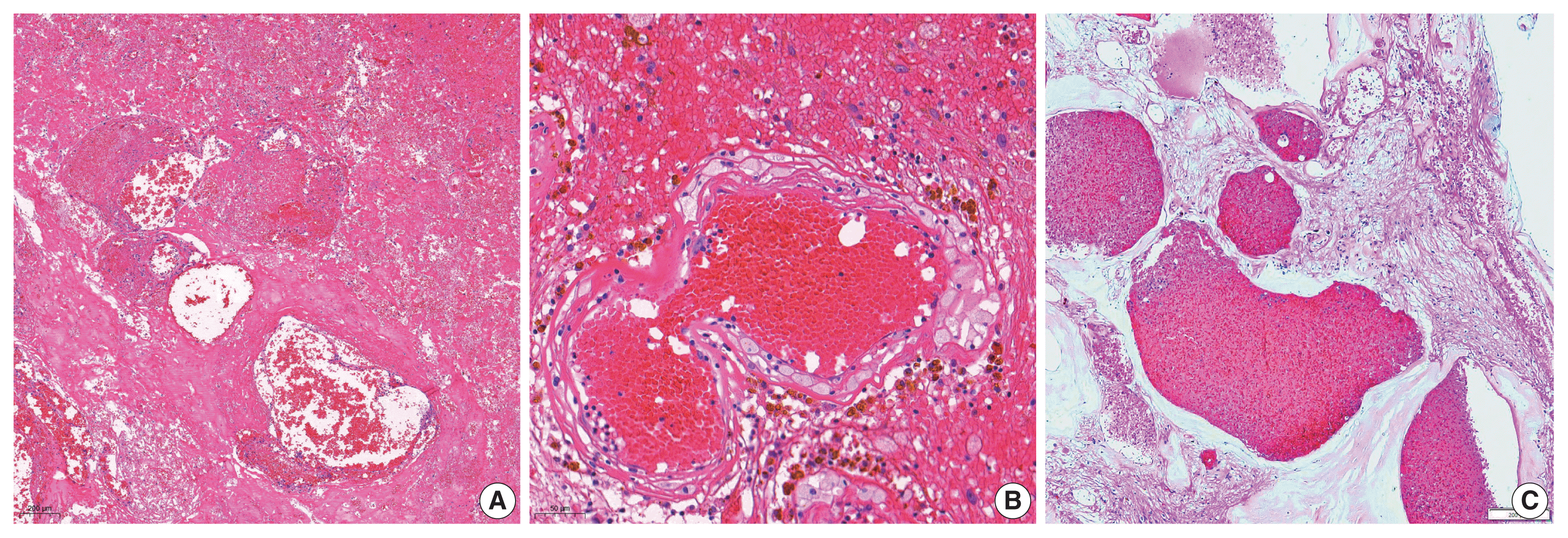
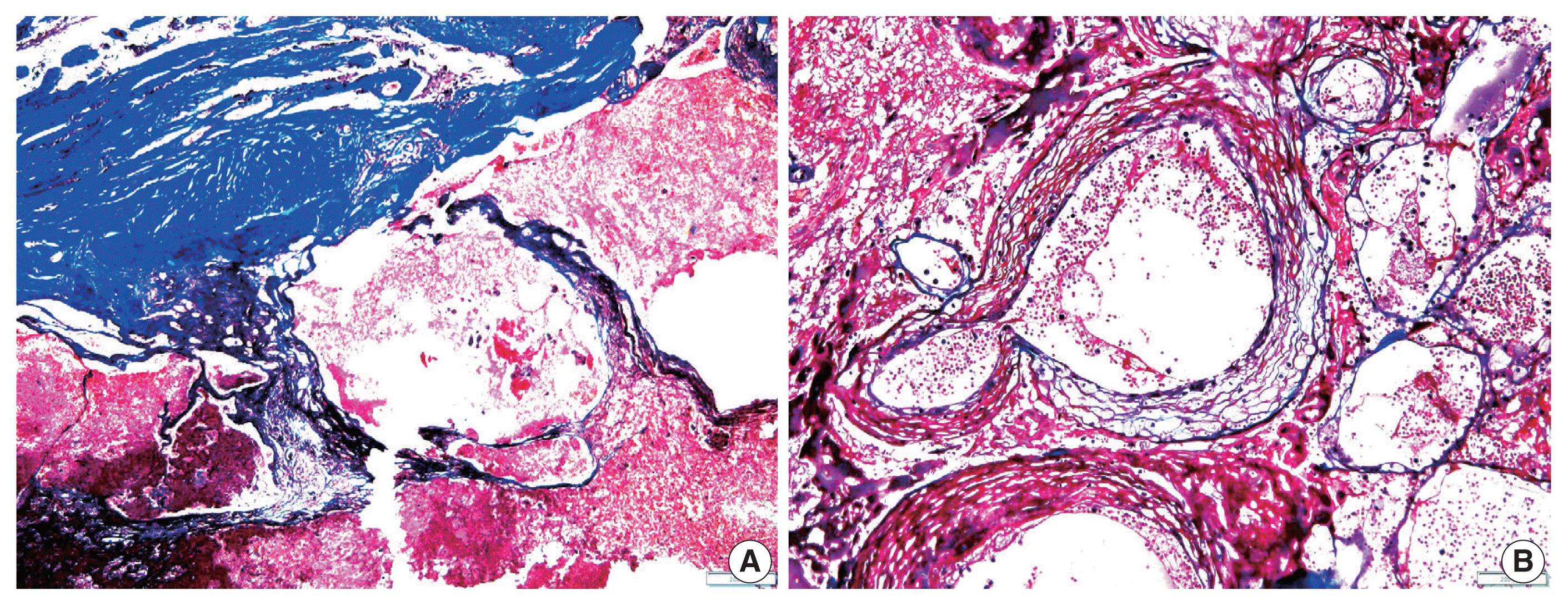
3. RIOH cases were divided into two groups based on previous type of radiation (GKS or conventional RT). Histologically, RIOH shows a hematoma-like area with a reduced number of hyalinized thin-walled vessels with fibrin and infiltrating foamy macrophages in the vessel walls. Conversely, de novo CH shows a thick, hyalinized wall without prominent macrophage infiltration (
Fig. 1). The results showed significantly shorter latency in the GKS group than in the conventional RT group (5.85 ± 4.06 years vs. 11.15 ± 8.27 years, p = .046). In addition, the RIOH in the GKS group had significantly thicker tumor wall than that in the conventional RT group (693.7 ± 565.7 μm vs. 406.9 ± 519.7 μm, p = .049) (
Fig. 2). Significant differences were not observed in age, tumor size of primary tumor, or RIOH. The primary tumor size did not differ between groups. However, the size of RIOH lesions tended to be larger in the GKS group than in the conventional RT group (p = .055). The multiplicity of RIOH was not significantly different between the groups. In terms of original pathology, the four groups based on preceding pathology were compared, and no significant differences were found in any of the clinicopathological parameters.
The results of the radiologic findings are summarized in
Table 4. All patients with GKS-induced RIOH and 11 patients with RT-induced RIOH showed perilesional edema on T2-weighted images. Seven patients in the GKS-induced RIOH group showed subacute stage hemorrhage on T1-weighted images, and 21 showed hemosiderin rim deposit on T2-weighted images. Similarly, nine patients in the RT-induced RIOH group showed subacute stage hemorrhage, and 11 cases showed hemosiderin rim. Significant difference in radiologic image findings including perilesional edema (p = .223), subacute stage hemorrhage (p = .059) and hemosiderin deposit in MR image (p = .679), was not observed between the GKS and RTx groups.
No significant differences were found in other parameters. In the GKS group, the mean age at the time of RIOH diagnosis was 46.9 years; the mean sizes of primary tumor and RIOH were 3.35 cm and 2.01 cm, respectively; and size difference between primary tumor and RIOH was 1.34 cm. In the conventional RT group, the mean age was 45.8 years; the sizes of primary tumor and RIOH measured 3.04 cm and 1.51 cm on average, respectively; and the size difference between primary tumor and RIOH was 1.53 cm. However, the size of RIOH lesions tended to be larger in the GKS group than in the conventional RT group (p = .055).
In terms of original pathology, the four groups based on preceding pathology were compared. The mean age in the AVM, brain tumor, metastasis, and other tumors groups was 43.1 years, 43.5 years, 57.2 years, and 52.7 years, respectively. The mean sizes of the primary tumor and RIOH were 3.87 cm and 2.06 cm in the AVM group, 2.85 cm and 1.50 cm in the brain tumor group, 2.92 cm and 2.20 cm in the metastasis group, and 2.85 cm and 1.75 cm in the other tumors group, respectively. The size difference was 1.81 cm, 1.35 cm, 0.72 cm, and 1.10 cm in the AVM, brain tumor, metastasis, and other tumors groups, respectively. The mean tumor wall thickness was 714.2 μm in the AVM group, 560.0 μm in the brain tumor group, 362.5 μm in the metastasis group, and 538.5 μm in the other tumors group. The average latency in the AVM, brain tumor, metastasis, and other tumors groups was 8.92 years, 7.55 years, 2.28 years, and 8.68 years, respectively. No significant difference was found in any of the clinicopathological parameters.
Three patients in the GKS group showed multiplicity, two in the conventional RT group, 1 in the AVM group, 2 in the brain tumor group, and two in the metastasis group; however, statistical difference was not found between the groups.
DISCUSSION
RIOH has been considered a sporadic form of de novo CH occurring as a late complication of cerebral radiation. RIOH occurs in children mainly after the use of cerebral radiation to treat medulloblastoma, glioma, or AVM or for prophylactic treatment of hematological malignancies such as acute lymphoblastic leukemia [
4,
5,
14,
15]. However, RIOH is rare in adults [
14,
16]. In a previous study with 84 cases of RIOH, the average age at diagnosis was 20.6 years, the median was 17 years, and the average latency to development of RIOH was 10.3 years, with a median of 8 years [
11]. One rare case of RIOH has been diagnosed at 52 years after RT [
17].
The pathophysiology of RIOH is not well-known; however, two hypotheses have been suggested: occult CHs that were previously present respond to radiation and become apparent; de novo formation of the lesion in response to radiation [
10]. The de novo mechanisms might include a series of processes, such as vascular injury, proliferation of the vascular wall, necrosis, and ischemia due to narrowing of the lumen. Previous studies showed increased vascular endothelial growth factor after exposure to radiation in rats, supporting this hypothesis [
18].
Patients with de novo CH can be treated with antiepileptic drugs and regular follow-up but are usually recommended to undergo surgery if possible in case the symptoms worsen or the size changes due to the risk of bleeding. In RIOH, which is regarded a sporadic form of de novo CM, surgical treatment is considered the standard treatment option following the treatment algorithm for de novo CM. However, if RIOH is considered a separate disease entity with different pathogenesis and clinical course than de novo CM, conservative treatment modalities could be considered a in addition to invasive surgical treatments, which could cause neurological side effects. Therefore, several studies have been conducted to compare the pathogenesis of RIOH and de novo CH, and several notable results were reported. Compared with de novo CH, RIOH was found to develop at younger age, symptoms at the time of diagnosis were milder, and tended to be more multifocal. However, the prevalence of a hemorrhagic event, the most fatal complication, was not significantly different [
11–
13].
Considering the hematoma-like area and infiltration of foamy macrophage, RIOH appears more likely to be an inactive hemangioma-like lesion, which might be closer to a recanalized cavitary hematoma induced by high-dose radiation than to vascular malformation [
6]. Therefore, we suggest the use of the term RIOH rather than RICH.
In the present study, we hypothesized that RIOH would show clinical and histological differences depending on treatment type or primary pathology. Based on the treatment type, the GKS group showed shorter latency. In previous studies, several factors affecting the duration of the latency period have been reported, including radiation dose > 30 Gy and RT before 10 years of age [
11,
14,
15,
19], with increased risk of hemorrhagic event [
9]. Hypothetically, the shorter latency observed in the GKS group might be due to its requirement of a higher dose in a smaller target area compared with conventional RT or focused damage that could accelerate tissue necrosis and tumorigenesis.
The present study had several limitations. First, the small sample size might have led to skewed statistical results. Second, although all available clinical, radiologic, and pathologic data were collected, some parameters might be inconsistent in retrospective analysis. Third, the subjects were older in the present study. As previously stated, RIOH occurs primarily in younger patients, however, in the present study, the mean age of the subjects was 46.6 years, which is an older age group compared with other reports (median 31.1 years [
12]; mean age at the time of radiation 10.4 years with mean latency of 10.3 years [
11]). Histopathological differences can exist in the RIOH of older subjects compared with younger subjects and could affect the results. However, only 37 patients were included in this study, most of whom were in their 30s or older, explaining why the results differ from those of previous studies. Further research with a larger cohort is needed to verify the results.
We hypothesized that the size of RIOH after conventional RT would be larger than after GKS because conventional RT is applied for larger lesions. However, the average size of the lesion tended to be larger in the GKS group, although the difference was not statistically significant. Furthermore, we hypothesized that the size difference between primary tumor and RIOH would be greater in the GKS group than in the RT group; however, significant difference was not observed. The repeated treatment applied in the same lesion, the error in the measurement of the radiological/pathological size, and the limitations of the present study described above might have affected these results.
In summary, RIOH after GKS tended to occur earlier and had thinner tumor wall than RIOH after conventional RT. The original pathology of RIOH had no effect on the histologic and clinical features of RIOH. These results suggest that the clinical course of RIOH differs based on type of treatment. Understanding the unique pathophysiology of RIOH, one of the most well-known complications of cerebral radiation, has become more important as the survival rate of brain tumor patients increases. Therefore, further investigation in the form of prospective studies with larger cohorts is required to elucidate more detailed clinical and histologic features of patients to provide appropriate medical management and predict the clinical course more accurately.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download