SEROUS NEOPLASMS
- Fallopian tube: STIC present, or mucosal HGSC present, or part or all of the fallopian tube is inseparable from tubo-ovarian mass.
- Ovary: both fallopian tubes are separate from ovarian mass, and no STIC or mucosal HGSC present in either fallopian tube.
- Tubo-ovarian: fallopian tubes and ovaries are unavailable for complete examination, and pathologic findings are consistent with extrauterine HGSC.
- Peritoneal (exceedingly rare): both fallopian tubes and ovaries are fully examined using a SEE-FIM (Sectioning and Extensively Examining the FIMbriated end) protocol, and no gross or microscopic evidence of STIC or HGSC present in either fallopian tube or ovary.
- These criteria classify approximately 80% of HGSCs as primary tubal.
- Obsolete terminology no longer recommended includes atypical proliferative serous tumor, serous tumor of low malignant potential, semimalignant serous tumor and non-invasive LGSC / micropapillary serous borderline tumor (the latter no longer considered definitionally synonymous with non-invasive LGSC).
SEROMUCINOUS CARCINOMA
NEW VARIANTS OF EPITHELIAL TUMORS
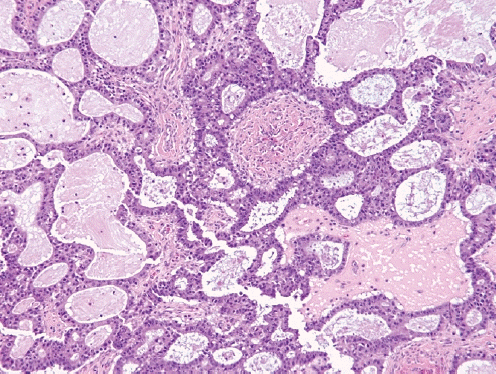
- Composed of multiple architectural patterns (tubular, glandular/pseudoendometrioid, ductal, papillary, solid), intraluminal eosinophilic colloid-like material, dense or vesicular chromatin, inconspicuous nucleoli and nuclear crowding, and lacking squamous or mucinous differentiation.
- Positive for GATA3, TTF1, CD10 (luminal) and PAX8, and negative for hormone receptors and WT1, with wild-type p53 expression.
- Usually unilateral and diagnosed at stage I in postmenopausal women.
- May arise from paraovarian mesonephric remnants or Müllerian carcinomas displaying secondary mesonephric transdifferentiation.
- May be associated with endometriosis, cystadenomas, adenofibromas, borderline tumors and LGSC.
- The most common molecular alterations include KRAS mutations, 1p loss and 1q gain, while NRAS or PIK3CA mutations are rare.
- Tumors with coexisting serous neoplasms show shared molecular alterations (KRAS or NRAS mutations).
- Clinical outcome is unknown due to rarity.
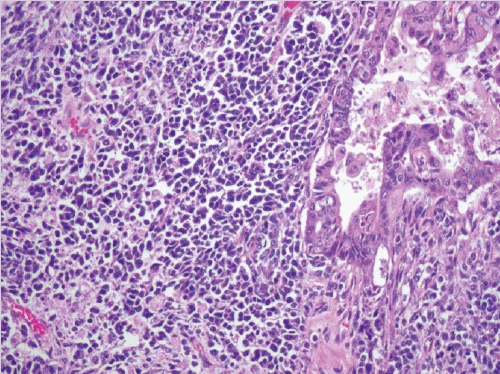
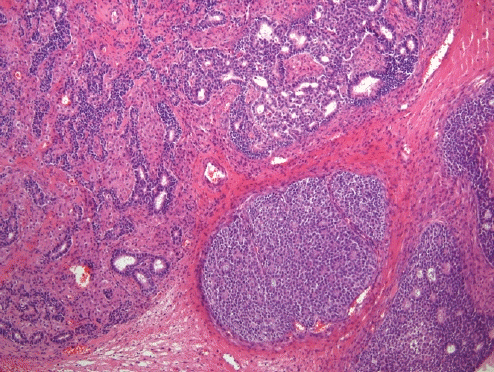
- A biphasic tumor composed of an undifferentiated carcinoma (sheet-like growth of monotonous, discohesive, round, rhabdoid to spindle cells with brisk mitoses, often necrosis and abundant tumor-infiltrating lymphocytes) and a differentiated (usually low grade endometrioid adenocarcinoma, rarely serous carcinoma) component, often with abrupt interface in between (Fig. 2).
- Undifferentiated areas are focally positive for EMA, pan-keratin and CK18, focally positive to negative for PAX8, and negative for hormone receptors and E-cadherin, with common loss of SMARCA4 (BRG1), SMARCA2 (BRM), SMARCB1 (INI1) or ARID1A, DNA mismatch repair deficiency in onethird of cases and typically wild-type p53 expression.
- Usually diagnosed at advanced stages, with pelvic and para-aortic lymph node involvement and poor prognosis.
- A biphasic neoplasm composed of high grade carcinomatous and sarcomatous elements.
- Now considered a variant of carcinoma rather than a true mixed epithelial-mesenchymal tumor.
- True mixed carcinomas are uncommon.
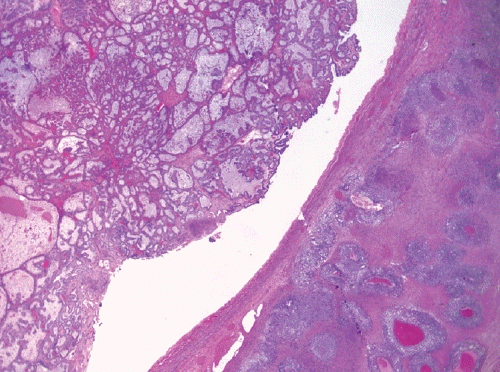
- Should only be diagnosed when at least two tumor types are clearly recognizable on hematoxylin-eosin-stained sections, with distinct morphologic and preferably immunophenotypic differences.
- Each histotype with their percentages should be reported (no minimum percentage requirement).
- Endometriosis-associated histotypes are most common, e.g. endometrioid and clear cell carcinomas (Fig. 3).
ANCILLARY TESTING
SEX CORD-STROMAL TUMORS
- DICER1-mutant tumors show somatic (~50%) or germline (69%) hotspot mutations in the RNase IIIb domain of DICER1, an endoribonuclease involved in microRNA processing and gene expression regulation.
- FOXL2-mutant tumors show c.402C>G (p.Cys134Trp) mutations that upregulate CYP19A1 encoding aromatase.
- Defined as a sex cord-stromal tumor with an admixure of female (adult-type or juvenile GCT) and male (Sertoli cell tumor or SLCT) elements.
- Most commonly composed of a predominant SLCT component and a smaller component of juvenile GCT, both expressing sex cordstromal markers, sometimes with shared DICER1 mutations.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download