Abstract
Notes
Ethics Statement
The study was approved by the Institutional Review Board of Asan Medical Center (IRB 2021-0180). Formal written informed consent was not required, with a waiver from the appropriate Institutional Review Board.
Availability of Data and Material
All data generated or analyzed during the study are included in this published article (and its supplementary information files).
Author Contributions
Conceptualization: HJK. Data curation: HJS. Investigation: HJS. Supervision: HJK. Visualization: HJS. Writing—original draft: HJS. Writing—review & editing: HJK, JK, KK, SY, JHL. Approval of final manuscript: all authors.
References
Fig. 1
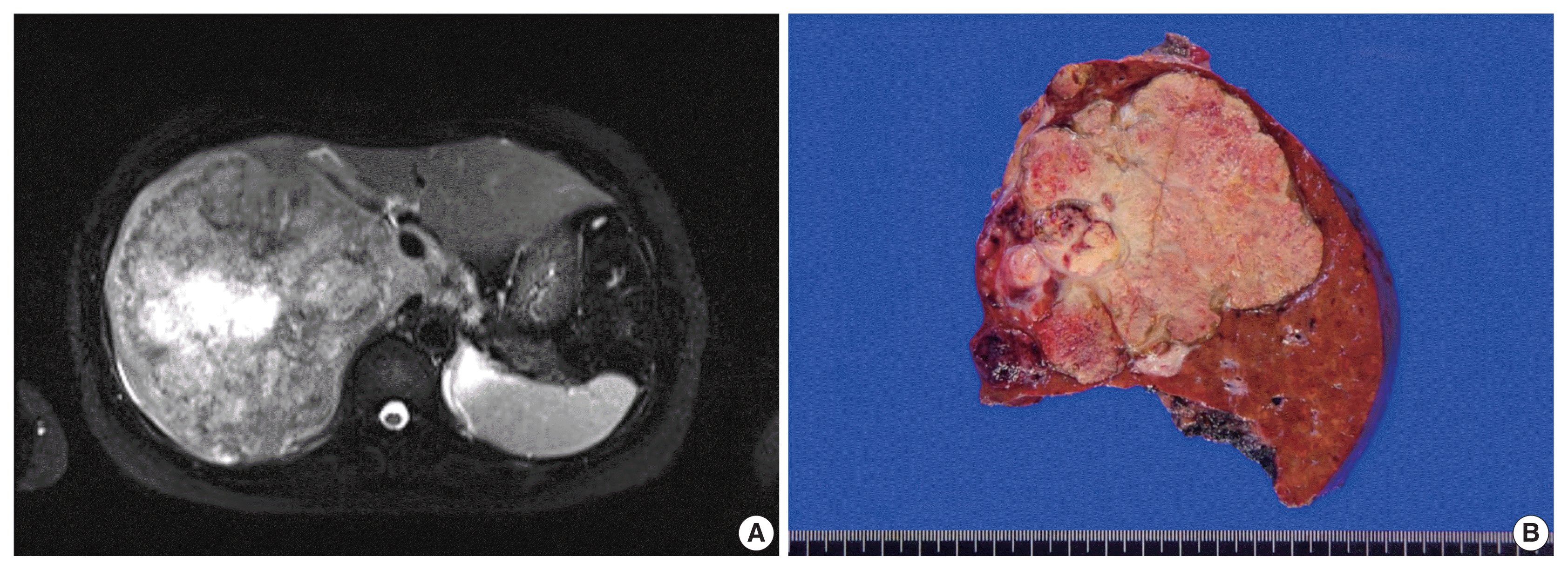
Fig. 2

Fig. 3

Table 1
| No. | Author | Age (yr)/Sex | Size (cm) | Treatment | Survival | Diagnosis | Metastasis | IHC (+) |
|---|---|---|---|---|---|---|---|---|
| 1 | Narita et al. [11] | 27/F | 11 | Resection, CTx | > 12 mo | Yolk sac tumor | No | AFP |
| 2 | Villaschi and Balistreri [12] | 28/F | 15 | Resection | - | Yolk sac tumor | No | SALL4 |
| 3 | Wong et al. [18] | 28/F | 15 | Resection | > 12 mo | Yolk sac tumor | No | AFP |
| 4 | Arai et al. [20] | 65/M | - | None | 45 days | Choriocarcinoma | No | hCG |
| 5 | Shi et al. [13] | 39/M | 12 | Resection, CTx | 6 mo | Choriocarcinoma | No | hCG |
| 6 | Shi et al. [13] | 45/M | 11 | CTx | 2 mo | Choriocarcinoma | Lung, brain | hCG |
| 7 | Shi et al. [13] | 48/M | 13 | CTx | 3 mo | Choriocarcinoma | Lung | hCG |
| 8 | Shi et al. [13] | 36/M | 10 | CTx | 5 mo | Choriocarcinoma | Peritoneum, adrenal gland | hCG |
| 9 | Shi et al. [13] | 40/M | 9 | Resection, CTx | 8 mo | Choriocarcinoma | No | hCG |
| 10 | Bakhshi et al. [21] | 40/M | - | Resection | 10 days | Choriocarcinoma | No | hCG |
| 11 | Sekine et al. [15] | 49/M | 10 | Resection | 2 mo | Choriocarcinoma | No | hCG |
| 12 | Makhmud et al. [8] | 54/F | - | Resection, CTx | - | Choriocarcinoma | Lung | hCG |
| 13 | Kohler et al. [14] | 64/M | 14.5 | Resection, CTx | 5 mo | Choriocarcinoma | Lung | hCG |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download