This article has been
cited by other articles in ScienceCentral.
Abstract
Hepatoid thymic carcinoma is an extremely rare subtype of primary thymus tumor resembling “pure” hepatoid adenocarcinomas with hepatocyte paraffin 1 (Hep-Par-1) expression. A 53-year-old man presented with voice change and a neck mass. Multiple masses involving the thyroid, cervical and mediastinal lymph nodes, and lung were detected on computed tomography. Papillary thyroid carcinoma was confirmed by biopsy, and the patient underwent neoadjuvant chemoradiation therapy. However, the anterior mediastinal mass was enlarged after the treatment whereas the multiple masses in the thyroid and neck decreased in size. Microscopically, polygonal tumor cells formed solid sheets or trabeculae resembling hepatocytes and infiltrated remnant thymus. The tumor cells showed immunopositivity for cytokeratin 7, cytokeratin 19, and Hep-Par-1 and negativity for α-fetoprotein. Possibilities of germ cell tumor, squamous cell carcinoma, and metastasis of thyroid papillary carcinoma were excluded by immunohistochemistry. This report on the new subtype of thymic carcinoma is the third in English literature thus far.
Keywords: Hepatoid carcinoma, Thymus, α-Fetoprotein, Hep-Par-1
Hepatoid thymic carcinoma (HTC) is a rare subtype of thymic carcinoma and has been inserted in the 4th edition
WHO classification of tumors of the lung, pleura, thymus and heart in 2015 [
1]. It was first described by Franke et al. [
2] in 2004 and only two cases have been reported until now [
2,
3]. This tumor was defined as a thymic carcinoma morphologically resembling hepatocellular carcinoma with immunoreactivity for hepatocyte paraffin 1 (Hep-Par-1) but immunonegativity for α-fetoprotein (AFP), differentiating from hepatoid adenocarcinoma (HAC) of other organs [
2]. Herein, we report an additional case of HTC.
CASE REPORT
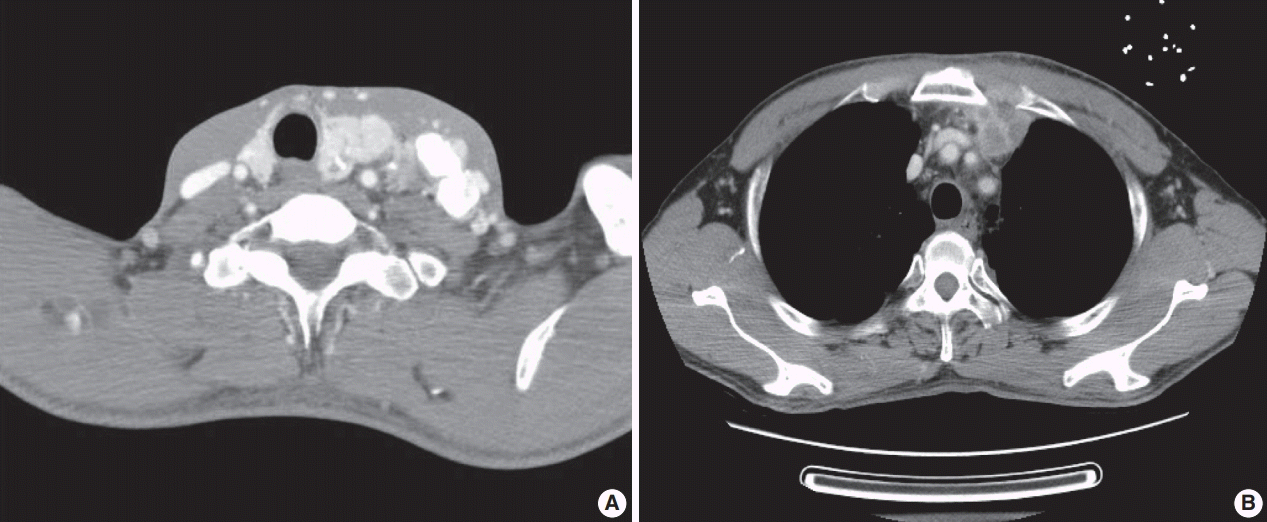
A previously healthy 53-year-old man presented with voice change and a neck mass. On the neck computed tomography (CT) and chest CT, multiple masses involving bilateral lobes of the thyroid gland, cervical lymph nodes and left mediastinum were detected (
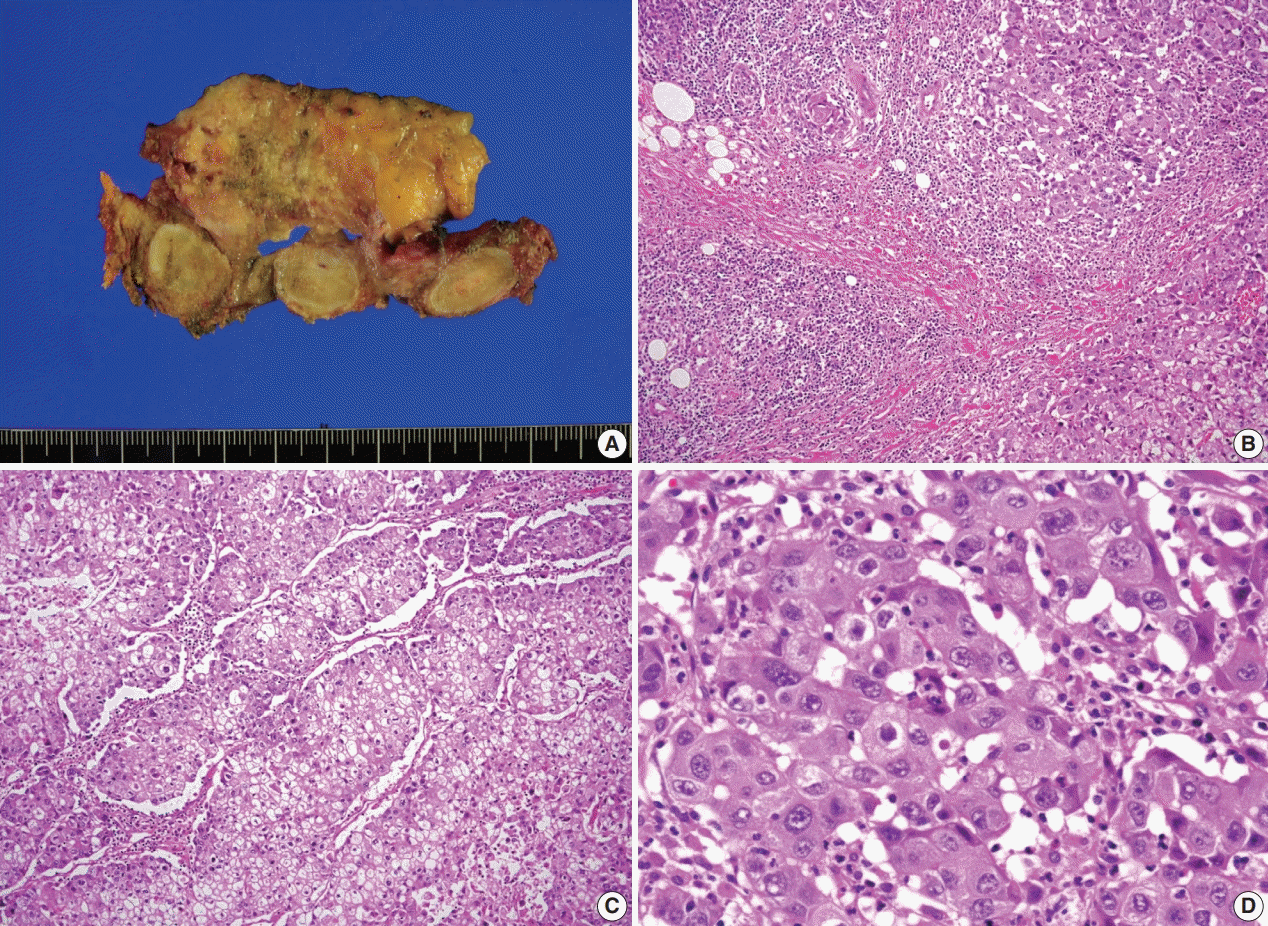
Fig. 1). Papillary thyroid carcinoma with metastases to neck lymph nodes were confirmed by core needle biopsies, and the mediastinal mass showed poorly differentiated carcinoma. The patient underwent neoadjuvant chemoradiation therapy (radiation therapy, 70 Gy/35 fractions; cisplatin based chemotherapy for 3 cycles) under the impression of long lasting papillary thyroid carcinoma with anaplastic change. However, the anterior mediastinal mass was enlarged after the treatment whereas the thyroid and neck masses decreased in size. The patient underwent total thyroidectomy with radical neck dissection and mediastinal mass excision. Both thyroid masses were severely calcified, and the histologic features were those of classic papillary thyroid carcinoma. An ill-demarcated anterior mediastinal mass measured 6 cm and showed heterogeneous, tan colored, and firm cut surface (
Fig. 2A). Microscopically, polygonal tumor cells formed solid sheets or trabeculae resembling hepatocytes and infiltrated remnant thymus. The tumor cells often showed clear cytoplasmic change (
Fig. 2B–
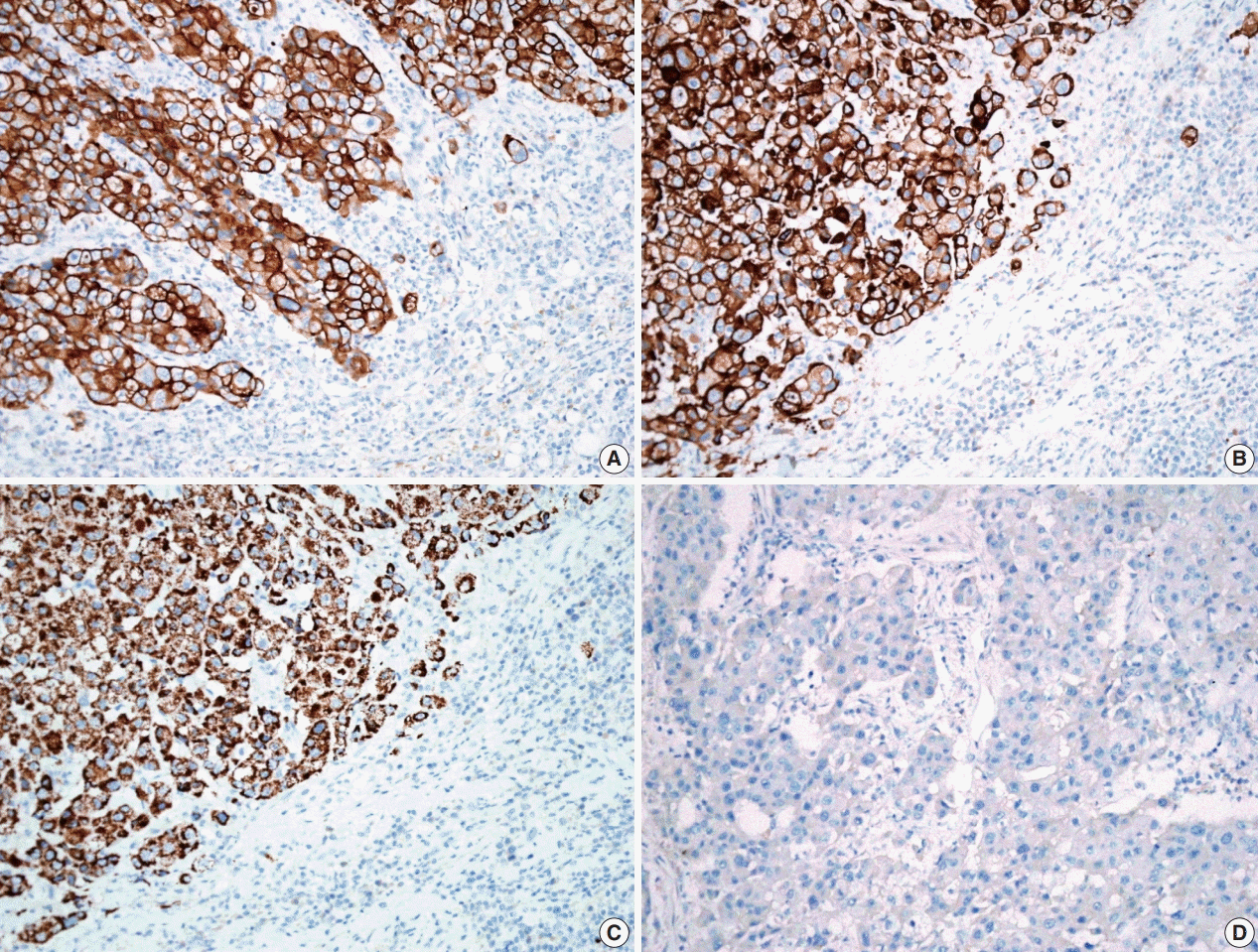
D), and immunopositivity for cytokeratin (CK) 7, CK 19 and Hep-Par-1 and negativity for AFP (
Fig. 3). Possibilities of metastatic hepatocellular carcinoma, germ cell tumor, squamous cell carcinoma, anaplastic thyroid carcinoma, and NUT carcinoma were excluded by immunohistochemistry (IHC). The results of IHC are summarized in
Table 1. The patient has been lost to follow-up after 6-month follow-up.
DISCUSSION
HTC is extremely rare and only two cases have been published in English literature thus far [
2,
3]. In brief, the patients were 70-year-old female and 34-year-old male. The sizes measured 18 cm and 6.6 cm. The patients underwent surgery with adjuvant chemoradiation therapy. The outcomes were not informative due to short follow-up periods or failure to follow-up. The results of IHC staining showed similar profiles in all cases as follows: CK 7 (+), CK 19 (+/–), Hep-Par-1 (+), and AFP (–) (
Table 2).
To exclude the possibility of anaplastic transformation of papillary thyroid carcinoma or poorly differentiated thyroid carcinoma, we performed IHC. Based on the results of IHC (paired box 8 [PAX-8] [–], thyroid transcription factor-1 [–], thyroglobulin [–]), we thought that this case was not of thyroid origin. It has been reported that immunoreactivity for PAX-8 in anaplastic thyroid carcinoma was 79% [
4]. Histologic features, adjacent involved thymic tissue and immunoreactivity for Hep-Par-1 supported the diagnosis of HTC.
HAC has been reported in many other organs including the stomach, gallbladder, lung, uterus, and urinary bladder, etc. [
5]. HTCs show distinct immunoprofiles that differentiate them from HAC. While 91.6% of HAC express AFP and 38.1% of HAC express Hep-Par-1, all HTCs are negative for AFP protein and positive for Hep-Par-1 although the cases are extremely rare. Hep-Par-1 is recognized as a mitochondrial antigen of hepatocyte and it is normally expressed in small intestinal epithelium and hepatocytes. Thus, it is highly sensitive (92%) in diagnosing hepatocellular carcinoma [
6]. However, our case had no hepatic mass, no evidence of hepatitis B and C, and was immunoreactive for CK 7 and CK 19, excluding the possibility of metastatic hepatocellular carcinoma.
HAC is a very aggressive neoplasm with metastasis at the time of diagnosis in a high proportion of patients and has a worse prognosis than more common types of tumors [
7]. More than half of patients died within the first 12 months [
5]. The 5-year survival rate of gastric HAC has been known to be significantly lower than that of conventional gastric cancer (9% vs. 44%, p= .001) [
7]. The 5-year survival rate of thymic carcinoma ranges from 30% to 70 %, depending on the initial stage of disease [
8]. HTC is also thought to have a poor prognosis, judging from the cases of HAC; however, it is hard to say definitively due to the small number of cases.
Histogenesis of this tumor is still unclear and the previous authors favored an endodermal origin rather than a germ cell origin [
2,
3], based on the hypothesis of endodermal origin of gastric and pulmonary hepatoid neoplasms [
9,
10]. Further study including genetic analysis is required to determine the histogenesis of this tumor.
In conclusion, we report this rare subtype of thymic carcinoma having peculiar characteristics of morphology and immunohistochemical features. In addition, being aware of this entity will contribute to collecting an adequate number of cases to lay the groundwork for identifying pathophysiology and establishing treatment in the near future.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download