Lung adenocarcinoma (LUAD) accounts for > 40% of lung cancers. It consists of several heterogeneous molecular subtypes defined by their oncogenic driver mutations [
1,
2]. Correctly classifying the molecular subtypes of LUAD is important in clinical practice because the presence of a certain driver mutation opens up therapeutic options that can improve the survival of patients with LUAD [
1,
2]. Epidermal growth factor receptor (
EGFR) mutations and anaplastic lymphoma kinase (
ALK) translocations are two of the most important oncogenic drivers in LUAD [
2]. Although they are considered to be mutually exclusive [
3], recent studies have shown that in some cases, albeit rarely, both
EGFR mutation and
ALK translocation are present in LUAD [
4–
9]. Most of those cases share several clinicopathological characteristics, including their advanced stage (III or IV) and a tumor morphology characteristic of LUAD due to translocated
ALK [
7]. In addition, in two cases, the immunohistochemistry (IHC) results suggested that the mutant-EGFR protein was expressed in the same tumor population as the ALK fusion protein [
5,
8]. However, here we report a case of early-stage LUAD with a concomitant
EGFR/ALK alteration in which the
ALK translocation was spatially segregated in a focal tumor area with a lepidic morphology.
CASE REPORT
A 63-year-old male patient who was a never-smoker presented with an incidentally found solitary pulmonary nodule. A pure ground-glass nodule (GGN) at the right lower lobe (RLL) had been detected 3 years previously, and a recent follow-up chest computed tomography (CT) exam had shown a GGN ~1.7 cm in diameter with a 0.5-cm solid portion (
Fig. 1A, B). A positron emission tomography–CT fusion scan showed mild uptake by the GGN but no other hypermetabolic lesion (
Fig. 1C). A lobectomy of the RLL was performed, and pathologic evaluation of the lesion confirmed a primary LUAD, 1.9×1.4×1.3 cm in size, with a predominantly acinar pattern but also associated lepidic and papillary patterns. There was no evidence of pleural invasion or nodal metastasis (pT1bN0, IA) (
Fig. 2).
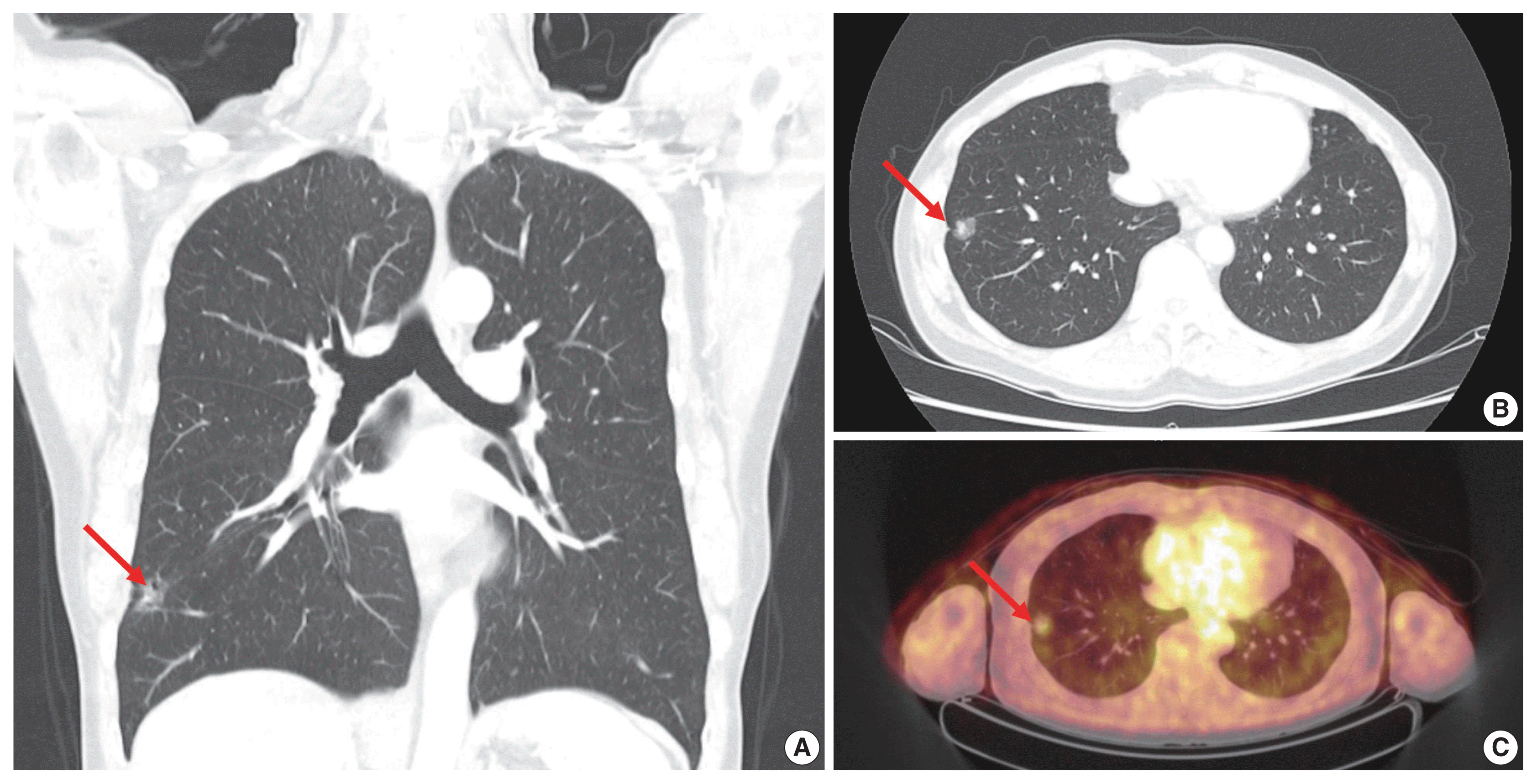 | Fig. 1Radiologic findings of the ground-glass nodule (GGN). (A, B) Coronal and axial computed tomography scan reveals a 1.7-cm GGN (arrows) with an inner solid portion measuring 0.5 cm in the right lower lobe of the lung. (C) The GGN (arrow) shows mild uptake on the positron emission tomography–computed tomography fusion scan, and no other hypermetabolic lesions are found. 
|
 | Fig. 2Pathologic features of the tumor. Microscopic evaluation of the tumor section shows lung adenocarcinoma with a predominantly acinar pattern (green circle) but also a lepidic portion (blue circle). Anaplastic lymphoma kinase (ALK) immunohistochemistry (IHC) reveals the focal expression of ALK within the part of the tumor showing lepidic growth. On ALK fluorescence in-situ hybridization (FISH), split signals (yellow circles) are seen within the ALK IHC-positive area whereas the ALK IHC-negative area is conformed as negative. H&E, hematoxylin and eosin. 
|
Tests for
EGFR mutation and
ALK translocation were carried out as part of the routine molecular work-ups for non–small cell lung cancer (NSCLC). A missense mutation in
EGFR exon 21 (L858R) was found by
EGFR PANAMutyper (PANAGENE, Daejeon, Korea). ALK IHC (clone D5F3, Cell Signaling Technology, Danvers, MA, USA) showed focal positivity within the part of the tumor characterized by lepidic pattern (
Fig. 2). Fluorescence in-situ hybridization (FISH) using a break-apart probe (Abbott Molecular Inc., Des Plaines, IL, USA) confirmed an
ALK translocation in the ALK-positive area and its absence in the ALK-negative area of the tumor.
Targeted next-generation sequencing (NGS) using Axen Cancer Panel 1 (Macrogen Inc., Seoul, Korea), comprising 88 cancer-related genes, was performed separately on the ALK IHC/FISH-positive and ALK IHC/FISH-negative tumor tissue. ALK IHC/ FISH-positive area was carefully microdissected from formalin-fixed, paraffin-embedded (FFPE) slide as shown in
Fig 2, and ALK IHC/FISH-negative tumor tissue was obtained from a separate FFPE block with no ALK IHC-positive portions. The tissue from ALK IHC/FISH-negative area harbored
EGFR L858R (variant allele frequency [VAF], 20.0%) and EGFR L833V (VAF, 19.5%) mutations (
Fig. 3A). The two
EGFR mutations were found in the same read, suggesting their presence in the same allele. Targeted sequencing of the ALK IHC/FISH-positive area revealed an
EML4 (echinoderm microtubule-associated protein-like 4)-
ALK fusion with breakpoints occurring at intron 18 of
EML4 and intron 19 of
ALK (
Fig. 3B), as well as the same
EGFR L858R and L833V mutations found in the ALK IHC/FISH-negative area, albeit at lower VAFs (5.9% and 3.4%, respectively) (
Fig. 3A).
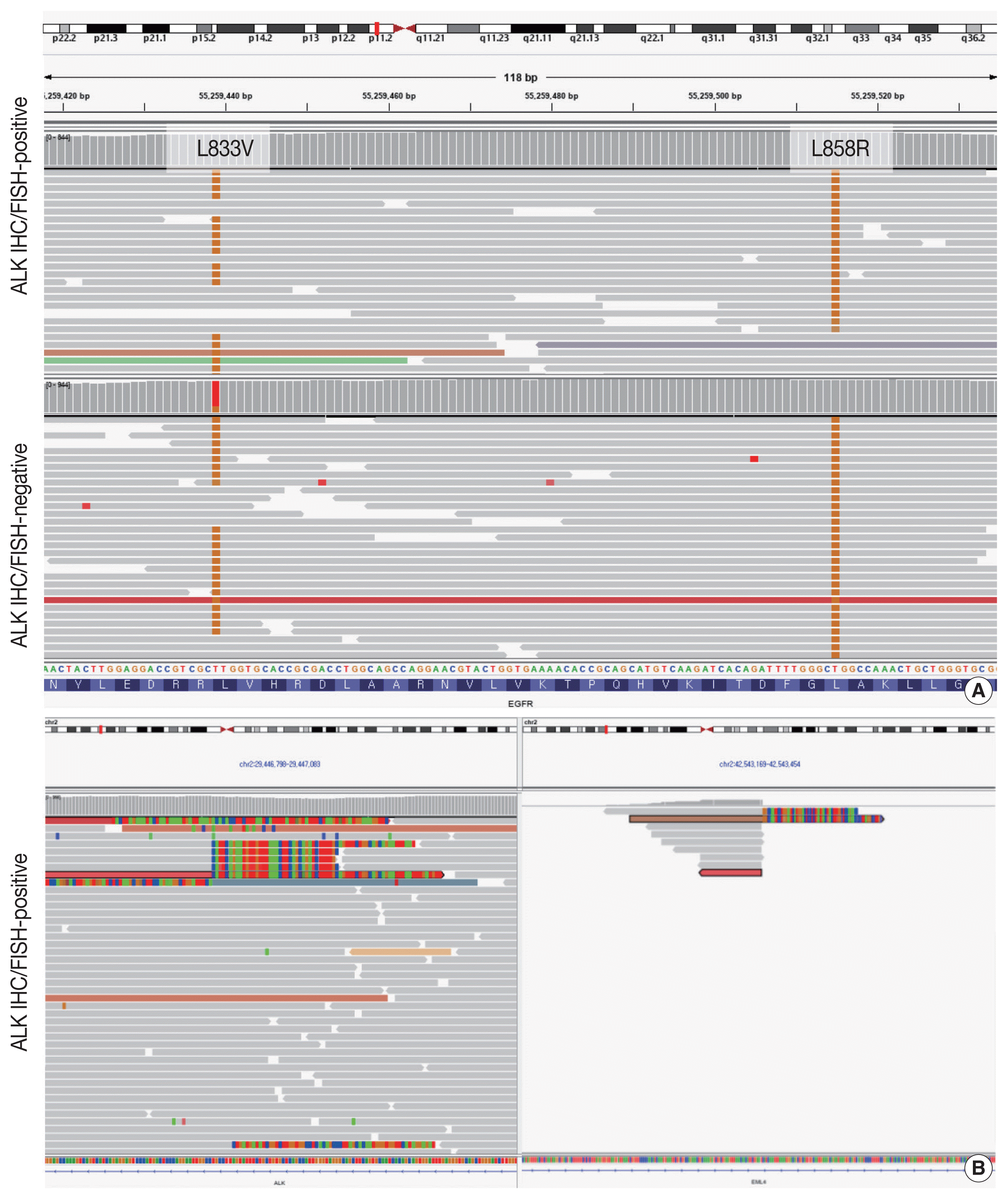 | Fig. 3Results of targeted sequencing of the anaplastic lymphoma kinase (ALK) immunohistochemistry (IHC)/fluorescence in-situ hybridization (FISH)–positive and -negative portions of the tumor. (A) Next-generation sequencing identified epidermal growth factor receptor (EGFR) L858R and L833V point mutations in both the ALK IHC/FISH-positive and -negative portions of the tumor, albeit with different variant allele frequencies. Tissues for targeted sequencing were obtained from separate slides to avoid potential contamination. Since both EGFR mutations were found in the same read, it is likely that they occurred in the same allele. (B) Soft clipped reads of ALK (left) and EML4 (echinoderm microtubule-associated protein-like 4) (right) with breakpoints at intron 18 of EML4 and intron 19 of ALK were found in the ALK IHC/FISH-positive portion of the tumor and suggested the fusion of the two genes. 
|
Go to :

DISCUSSION
Here we report a case of early-stage LUAD with concomitant EGFR and ALK alterations. In the mutated EGFR background of the tumor, a focal area with an additional ALK translocation was identified by IHC and FISH. NGS confirmed that the ALK-positive lesion harbored dual EGFR and ALK alterations and that identical EGFR mutations were shared by both ALK-positive and -negative portions of the tumor, suggesting the divergence of an EGFR/ALK co-altered subclone from the original EGFR-mutant clone.
Both
EGFR and
ALK alterations are major driver mutations in LUAD, and was considered to be mutually exclusive [
3]. However, rare cases with concomitant
EGFR and
ALK alterations have been reported [
4–
9]. The prevalence of concomitant
EGFR mutation and ALK translocation among NSCLC patients was estimated to be 0.9% [
6]. Although our case is not the first report of a LUAD carrying both
EGFR and
ALK alterations, its clinicopathological features were unique.
In previous reports of LUAD with concomitant
EGFR/ALK alterations, the tumors were often clinically advanced [
4,
5,
7–
10], whereas in our patient the tumor was stage IA LUAD. These findings suggest that co-alterations of
EGFR and
ALK can occur in the very early phase of tumorigenesis. Pathologic evaluation of the tumor revealed the lepidic growth pattern of the ALK-positive portion whereas the pattern in the ALK-negative portion was predominantly acinar. LUADs with concomitant
EGFR/ ALK alterations frequently exhibited a solid, cribriform, or micropapillary patterns [
7], while our case suggests that LUAD with co-altered
EGFR/ALK can also include low-grade histologic patterns. These findings demonstrate that LUAD with concomitant
EGFR/ALK alterations is not limited to a single morphology but may, instead, be characterized by a wide histologic spectrum.
Despite previous reports of concomitant
EGFR/ALK co-altered LUADs, the spatial and sequential contexts of the two alterations are poorly understood. Yang et al. [
5] identified seven cases of LUAD with co-localized expression of mutant
EGFR and
ALK, determined in serial sections of the FFPE tumor samples. Their observations evidenced the intra-tumor and potential intracellular coexistence of
EGFR and
ALK alterations in certain LUADs. Our case also shows that
EGFR and
ALK can be co-altered in LUAD within the same tumor population, as verified by targeted sequencing. Moreover, the sequencing results provided additional insights, by revealing combined L858R and L833V mutations in the
EGFR gene. This is an extremely rare finding, with only a single case identified among 783 NSCLCs analyzed [
11]. Thus, it is highly unlikely that the
EGFR L858R and L833V mutations in the ALK-positive and ALK-negative areas occurred independently, de novo; rather, a more plausible scenario is that the two-point mutations arose as a single first hit, followed by
ALK translocation in a subclone stemming from the main tumor that subsequently followed a divergent evolution.
The spatial distribution of the mutant
EGFR and
ALK fusion proteins in two advanced LUAD cases was previously analyzed using IHC, and the homogeneous co-localization of the
EGFR/ALK alterations was determined in both [
8]. This finding contrasts with the present case, in which the
ALK translocation was likely to have been a sub-clonal event that gives rise to LUAD with co-altered
EGFR/ALK. Further studies are needed to verify that, in tumorigenesis of LUAD with concomitant
EGFR/ALK alterations, the two events were indeed consecutive.
Nevertheless, the possibility of collision tumor cannot be completely ruled out; current extraction-based, bulk sequencing cannot verify that two EGFR mutations and ALK translocation exist in the very same tumor cell. However, we performed NGS after careful microdissection of the tumor area showing lepidic pattern, where virtually every tumor cells stained positive for ALK protein. This implies that the likelihood of the presence of EGFR-only-mutated tumor cell population within the micro-dissected area would be extremely low. Recent advances in single-cell sequencing technology would help deciphering the clonal events in single-cell resolution.
There is currently no consensus regarding the treatment of LUAD with concomitant
EGFR/ALK alterations, and conflicting results on the efficacy of EGFR tyrosine kinase inhibitors (TKI) versus ALK inhibitors as first-line treatment have been reported [
4–
9]. Won et al. [
4] found that the clinical outcome of patients treated with ALK inhibitors was substantially better than that of patients treated with EGFR TKIs. However, based on their own multi-center retrospective analysis and review of published data, Schmid et al. [
9] concluded that
EGFR/ALK co-altered LUADs were more likely to be resistant to ALK inhibitors than to EGFR TKIs. Yang et al. [
5] attempted to explain these conflicting results by considering the phosphorylation status of EGFR and ALK as potential predictive biomarkers of treatment response, but the number of patients analyzed was too small to draw definitive conclusions.
In our patient, although kinase inhibitor treatment was not needed, as the tumor was small enough to be locally excised, it is reasonable to assume that the treatment response would have been better with an EGFR TKI than with an ALK inhibitor, because the ALK translocation was present only in a subclone of the tumor. Identification of the initial genetic alteration may therefore aid in determining the primary driver mutation in EGFR/ALK co-altered LUAD, even in advance-stage tumors, as it may, in turn, lead to optimal treatment selection.
In summary, we report a case of early-stage, EGFR-mutated LUAD with a focal EGFR/ALK co-alteration. Our study suggests that concurrent mutations of EGFR and ALK can arise via divergent tumor evolution, even in the relatively early phases of tumorigenesis.
Go to :








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download