Table 1
| Case No. | Study | Year | Age (yr) | Sex | Site | Clinical diagnosis | Size (cm) | Treatment | F/U period | Prognosis | Positive IHC | Negative IHC | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Current case | 2020 | 33 | M | Lower extremity (Rt. knee) | Epidermal cyst | 2.0 | Excision | 8 mo | NROMD | S100 (diffuse, luminal component; patchy, solid component), EMA (patchy, luminal component; diffuse, solid component), CK7, CK5/6 (patchy), CD117 (patchy), EpCAM (patchy), CEA (intra-luminal), p63 (rare) | CK20, GCDFP-15, ER, calponin, SMA |
History of intracranial hemorrhage due to arteriovenous malformation No evidence of other malignancies |
| 2 | Requena et al. [1] | 1998 | NA | NA | Lower extremity | NA | NA | Excision | NA | NA | NA | NA |
First description Reported as mainly women with a mean age of 44 years (range, 20 to 55) Two cases of recurrent tumor after incomplete excision |
| 3 | NA | NA | Lower extremity | NA | NA | Excision | NA | NA | NA | NA | |||
| 4 | NA | NA | Lower extremity | NA | NA | Excision | NA | NA | NA | NA | |||
| 5 | NA | NA | Upper extremity | NA | NA | Excision | NA | NA | NA | NA | |||
| 6 | NA | NA | Pubis | NA | NA | Excision | NA | NA | NA | NA | |||
| 7 | Adamski et al. [5] | 2005 | 37 | M | Lower extremity (Lt. popliteal fossa) | NA | 2.0 | Excision | 2 yr | NROMD | CK7 | CK20, GCDFP-15, S100 | - |
| 8 | Fernandez-Flores et al. [4] | 2007 | 62 | F | Lower extremity (Lt. popliteal fossa) | NA | 0.8 | Excision | NA | NROMD | CK AE1/AE3, CAM 5.2, CK7, EMA, ER (2+, 1%–5% cells), c-erbB-2 (2+, 50%–75% cells), p53 (1+, 1%–5% cells), S100 (2+, 50%–75% cells) | CK20, CEA, PR, GCDFP-15, CD15, SMA | - |
| 9 | Rutten et al. [2] | 2009 | 48 | M | Lower extremity (Rt. thigh) | Dermatofibroma (histiocytoma) | - | Excision | 18 yr | NROMD | CK MNF116, CK AE1/AE3, CAM 5.2, CK7, CEA (more prominent in the ductal structures), EMA (more prominent in the ductal structures) | CK20, GCDFP-15, S100, α-SMA, MSA, calponin, CD68, vimentin | - |
| 10 | 51 | F | Upper extremity (Rt. forearm) | Cyst | - | Excision | NA | NA | - | ||||
| 11 | 44 | F | Back | Dermatofibroma (histiocytoma) | - | Excision | 13 yr | NROMD | - | ||||
| 12 | 77 | F | Neck | NA | - | Excision | NA | NA | Recurrent tumor after incomplete excision | ||||
| 13 | 32 | F | Lower extremity (Rt. thigh) | Dermatofibroma (histiocytoma) | - | Excision | 9 yr | NROMD | - | ||||
| 14 | 42 | F | Lower extremity (Rt. thigh) | Dermatofibroma (histiocytoma) | - | Excision | NA | NA | - | ||||
| 15 | 34 | F | Upper extremity (Rt. arm) | Dermatofibroma (histiocytoma) | - | Excision | 11 yr | NROMD | - | ||||
| 16 | 40 | M | Upper extremity (Lt. forearm) | Dermatofibroma (histiocytoma) | - | Excision | NA | NA | - | ||||
| 17 | 23 | M | Lower extremity (Lt. calf) | NA | - | Excision | NA | NA | - | ||||
| 18 | 20 | F | Rt. buttock | Cyst | - | Excision | NA | NA | No evidence of other malignancies | ||||
| 19 | 67 | F | Lt. preauricular area | BCC vs. adnexal tumor | - | Excision | 8 yr | NROMD | - | ||||
| 20 | 60 | M | Lower extremity (Rt. foot dorsum) | Dermatofibroma (histiocytoma) | - | Excision | 4 yr | NROMD | - | ||||
| 21 | 54 | F | Upper extremity (acral) | NA | - | Excision | NA | NA | - | ||||
| 22 | 49 | F | Lower extremity (Lt. thigh) | Dermatofibroma (histiocytoma) | - | Excision | 4 yr | NROMD | - | ||||
| 23 | 59 | F | Lt. shoulder | Dermatofibroma (histiocytoma) | - | Excision | NA | NA | - | ||||
| 24 | 28 | M | Lower extremity (Rt. lower leg) | NA | - | Excision | NA | NA | - | ||||
| 25 | 54 | M | Upper extremity (Rt. hand) | NA | - | Excision | NA | NA | - | ||||
| 26 | 50 | F | Upper extremity (Rt. hand) | NA | - | Excision | NA | NA | - | ||||
| 27 | 70 | M | Rt. trunk | Long-standing lesion | - | Excision | 6 yr | NROMD | - | ||||
| 28 | 64 | F | NA | Dermatofibroma (histiocytoma) | - | Excision | 5 yr | NROMD | - | ||||
| 29 | 40 | F | Lower extremity (Lower arm) | NA | - | Excision | 5 yr | NROMD | - | ||||
| 30 | 64 | M | Upper back | Dermatofibroma (histiocytoma) or cyst | - | Excision | 3 yr | NROMD | - | ||||
| 31 | 54 | M | Lower extremity (Lower arm) | NA | - | Excision | 3 yr | NROMD | - | ||||
| 32 | 44 | F | Upper extremity (Lt. thumb) | NA | - | Excision | 2 yr | NROMD | - | ||||
| 33 | 36 | F | Lower extremity (Lt. anterior thigh) | Dermatofibroma (histiocytoma) | - | Excision | NA | NA | - | ||||
| 34 | 26 | F | Lower extremity (Lt. posterior leg) | Fibroma | - | Excision | NA | NA | - | ||||
| 35 | Arps et al. [3] | 2015 | 41 | F | Lower extremity (leg) | Epidermal inclusion cyst | 0.6 | Excision | NA | NROMD | S100 (diffuse in three cases, patchy in one case), CD117 (diffuse in two cases, patchy in one case), CK5/6, CK7, EpCAM, CEA (luminal), EMA (luminal), p63 (rare) | CK20, GCDFP-15, ER, PR, calponin, SMA | - |
| 36 | 32 | F | Upper extremity (elbow) | NA | 0.5 | Excision | NA | NA | - | ||||
| 37 | 35 | M | Lower extremity (leg) | NA | 0.5 | Excision | NA | NROMD | - | ||||
| 38 | 59 | F | Upper extremity (arm) | NA | 0.4 | Excision | NA | NA | - | ||||
| 39 | 61 | M | Upper extremity (arm) | Mobile nodule | 0.7 | Excision | NA | NA | - | ||||
| 40 | 31 | M | Lower extremity (leg) | Dermatofibroma | 1.2 | Excision | NA | NA | - | ||||
| 41 | Yokota et al. [7] | 2017 | 39 | F | Upper extremity (Rt. forearm) | NA | 0.5 (clinical) | Excision | 15 mo | NROMD | CK5/6, CK7, CA15-3, CA125, CD117, S100 (partially), p53 (partially), p63 (partially) | CK20, calponin, GCDFP-15, mammaglobulin, MUC1, ER, AR, D2–40 | Stable in size for more than 10 years, no evidence of other malignancies |
| 42 | Bogner et al. [6] | 2018 | 65 | M | Lt. lateral neck | NA | 0.6 (clinical) | Excision | 3 mo | NROMD | CK7 (strong), EMA (strong), CAM 5.2 (strong), EpCAM (lesser degree), CEA (in some of the lumina), p63, p40 | CK20, D2–40, TTF-1, CDX-2, hepatocyte antigen, PSA, PSAP, calponin, S100 | No evidence of other malignancies |
F/U, follow up; IHC, immunohistochemical staining; Rt., right; Lt., left; NROMD, no recurrence or metastatic disease; EMA, epithelial membrane antigen; CK, cytokeratin; EpCAM, epithelial cell adhesion molecule; CEA, carcinoembryonic antigen; GCDFP-15, gross cystic disease fluid protein-15; ER, estrogen receptor; SMA, smooth muscle actin; MSA, muscle specific antigen; NA, data not available; S100, S-100 protein; PR, progesterone receptor; BCC, basal cell carcinoma; MUC1, mucin1; AR, androgen receptor; TTF-1, thyroid transcription factor 1; PSA, prostate-specific antigen; PSAP, prostatic acid phosphatase; +, >50% of tumor cells are positive; patchy, 25%–50% of tumor cells are positive; rare, < 1% of tumor cells are positive.
CASE REPORT
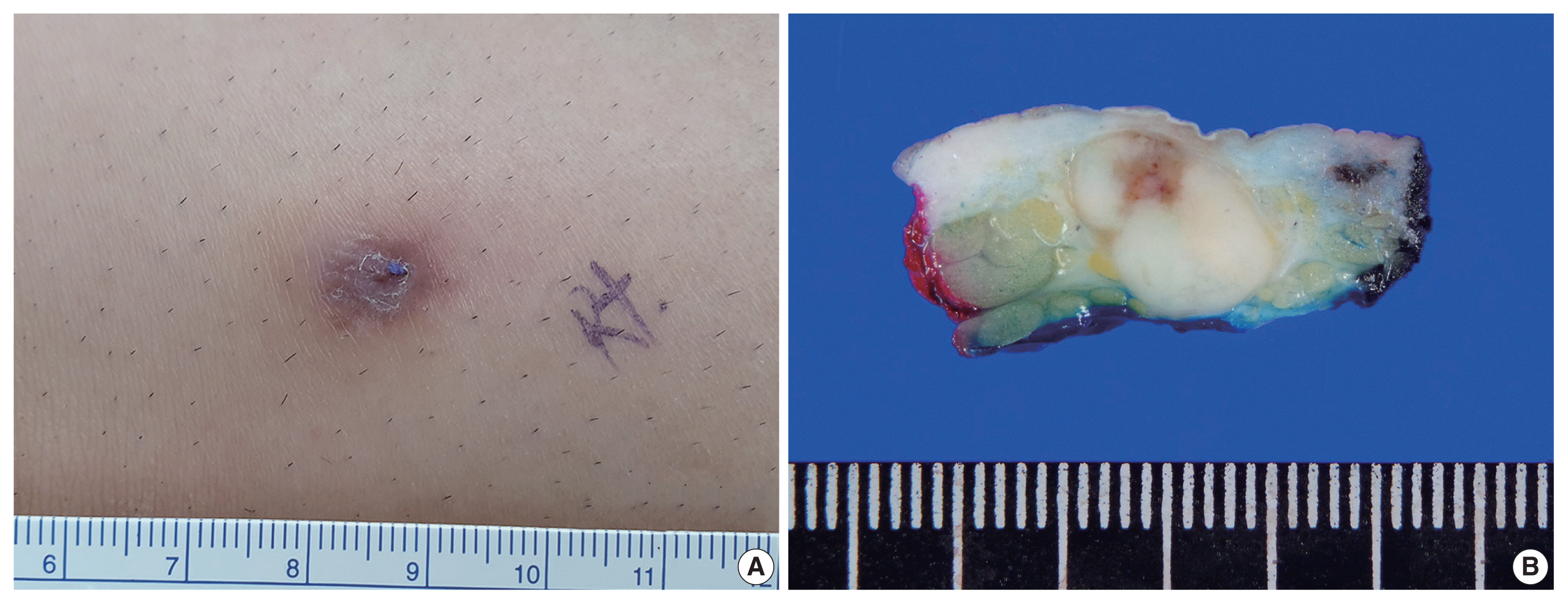
 | Fig. 2Histopathologic features of the mass. (A) The mass exhibited a predominantly solid component (90%) and a predominantly cribriform component (10%, inside the green line). (B) The predominantly solid component revealed pleomorphic nuclei. (C) The predominantly cribriform component had similar cytologic features to those of the solid component and demonstrated many prominent small lumina with a thin thread-like intraluminal bridge (cribriform pattern) along with an occasional eosinophilic substance. (D) The intraluminal eosinophilic substance with periodic acid–Schiff reaction. (E) Multifocal lymphoid aggregates at the periphery. (F) Infiltrative tumor clusters at the periphery. S, predominantly solid component; L, predominantly luminal component. |
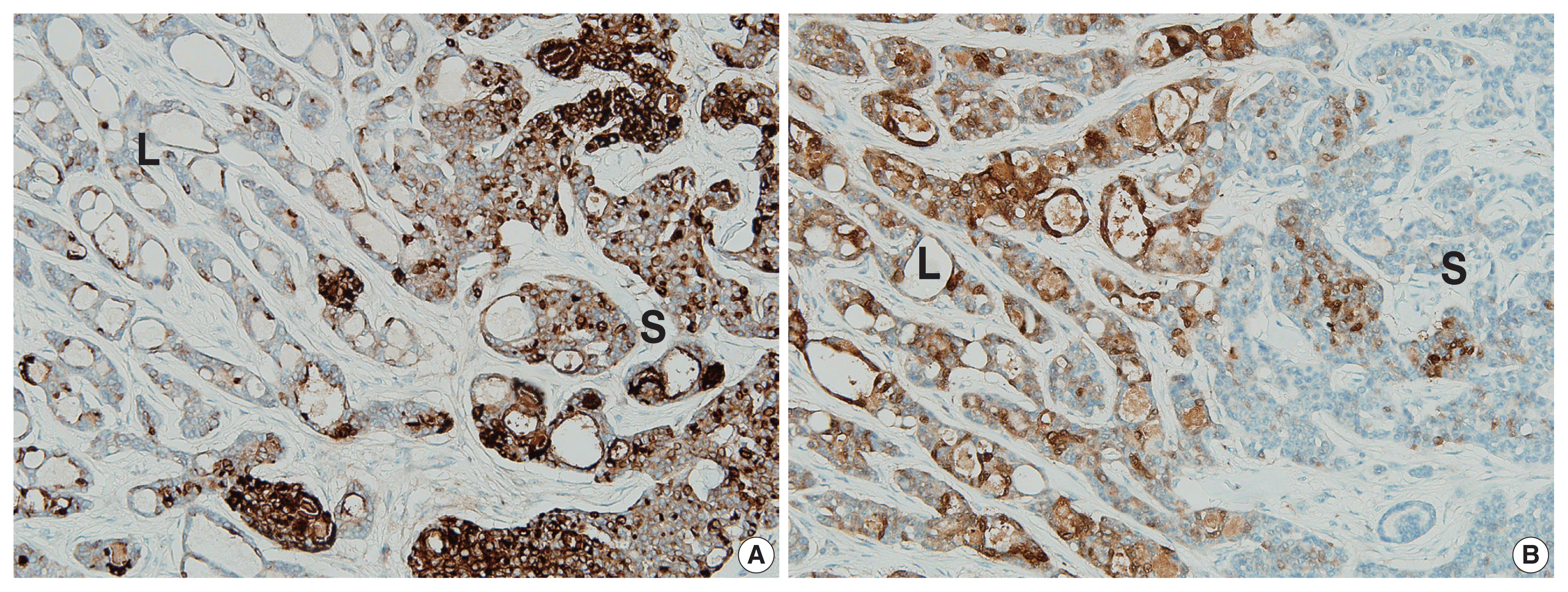 | Fig. 3Results of immunohistochemical (IHC) staining. (A) IHC for epithelial membrane antigen: diffuse immunopositivity for the predominantly solid component (on the right) and focal immunopositivity for the predominantly luminal component (on the left). (B) IHC for S-100 protein: diffuse immunopositivity for the predominantly luminal component (on the left) and focal immunopositivity for the predominantly solid component (on the right). S, predominantly solid component; L, predominantly luminal component. |
DISCUSSION
Table 2
| Cribriform carcinoma | Adenoid cystic carcinoma | Secretory carcinoma | Tubular adenoma | |
|---|---|---|---|---|
| Architecture |
Usually well-circumscribed Mixed variable portion of solid and cribriform |
Poorly circumscribed Composed of lobules, islands, and cords of basaloid cells with numerous cystic and ductular spaces |
Intradermal, circumscribed Back-to-back proliferation of tubules and microcysts |
Well circumscribed Variable sized tubules with attenuated epithelium |
| No back-to-back appearance | Cuboidal cells | Micro-papillae, and focal intraluminal bridging | ||
| No cuboidal cells | Sclerotic stroma | Paucicellular fibrous stroma | ||
| Desmoplastic stroma | Recognition of myoepithelial layer | |||
| Intra-(pseudo) luminal substance | Eosinophilic substance with PAS reaction | Mucin or basement membrane material that stains with mucicarmine, Alcian blue, and colloidal iron | Conspicuous intraluminal secretions | Eosinophilic proteinaceous material |
| Nuclei | Pleomorphic | Uniform | Mildly pleomorphic | Uniform |
| Mitosis | Rare | Rare | Rare to few | Absent |
| Perineural invasion | Absent | Present, frequent | Absent | Absent |
| Immunohistochemical staining | Variable CK (MNF116, AE1/AE3, CAM5.2, and CK7) | EMA and monoclonal CEA | S100 protein, mammaglobin and STAT5A | HMFG-1 and GCDFP-15 |
| EpCAM | S100, p63, GFAP, SMA, MSA and calponin: often stain peripheral cells (myoepithelial differentiation) | NTRK3: variable | EMA and CEA: luminal cells | |
| CD117, S100, and p63: variable | S100 and SMA: myoepithelial cells | |||
| CEA, EMA: highlight ductal component | ||||
| Reference | [11,12] | [12,13] | [12,14] | [3,12,13] |
PAS, periodic acid-Schiff; CK, cytokeratin; EMA, epithelial membrane antigen; CEA, carcinoembryonic antigen; S100, S-100 protein; GCDFP-15, gross cystic disease fluid protein-15; EpCAM, epithelial cell adhesion molecule; GFAP, glial fibrillary acidic protein; SMA, smooth muscle actin; MSA, muscle specific antigen.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download