MATERIALS AND METHODS
Patients
Mutational analyses for TERT promoter and BRAF V600E mutations
Statistical analysis
RESULTS
Demographic and clinicopathologic characteristics
Table 1.
| Characteristic | No. (%) (n = 724) |
|---|---|
| Age at diagnosis (yr) | 45.9 ± 13.0 |
| < 55 | 531 (73.3) |
| ≥ 55 | 193 (26.7) |
| Sex | |
| Female | 528 (72.9) |
| Male | 196 (27.1) |
| Tumor size (cm) | |
| ≤ 1.0 | 518 (71.5) |
| > 1.0 | 206 (28.5) |
| Surgical procedure | |
| Lobectomy | 504 (69.6) |
| Total thyroidectomy | 191 (26.4) |
| Isthmusectomy | 29 (4.0) |
| Histologic types | |
| Classic | 490 (67.7) |
| Classic with tall cell features | 83 (11.5) |
| Classic encapsulated | 46 (6.4) |
| Tall cell variant | 49 (6.8) |
| Warthin-like variant | 15 (2.1) |
| Infiltrative follicular variant | 10 (1.4) |
| Invasive encapsulated follicular variant | 6 (0.8) |
| Diffuse sclerosing variant | 8 (1.1) |
| Oncocytic variant | 8 (1.1) |
| Solid variant | 5 (0.7) |
| Hobnail variant | 3 (0.4) |
| Cribriform-morular variant | 1 (0.1) |
| Extrathyroidal extensiona | |
| Absent | 278 (38.4) |
| Minimal (microscopic) | 405 (55.9) |
| Gross (strap muscle invasion, pT3b) | 30 (4.1) |
| Gross (tracheal, esophageal or recurrent laryngeal nerve invasion, pT4a) | 11 (1.5) |
| Pathologic T categorya | |
| pT1 | 651 (89.9) |
| pT2 | 30 (4.1) |
| pT3 | 32 (4.4) |
| pT4 | 11 (1.5) |
| Lymph node metastasisa | |
| Absent (pN0) | 315 (43.5) |
| Central lymph node (pN1a) | 346 (47.8) |
| Lateral lymph node (pN1b) | 63 (8.7) |
| ATA recurrence risk | |
| Low risk | 241 (33.3) |
| Intermediate risk | 358 (49.4) |
| High risk | 125 (17.3) |
| AJCC cancer staginga | |
| Stage I | 623 (86.0) |
| Stage II | 98 (13.5) |
| Stage III | 3 (0.4) |
| Stage IV | 0 |
| BRAF V600E mutation | |
| Absent | 108 (14.9) |
| Present | 616 (85.1) |
| TERT promoter mutation | |
| Wild | 704 (97.2) |
| C228T mutation | 14 (1.9) |
| C250T mutation | 2 (0.3) |
| C216T variant | 4 (0.6) |
Frequency of TERT promoter and BRAF V600E mutations
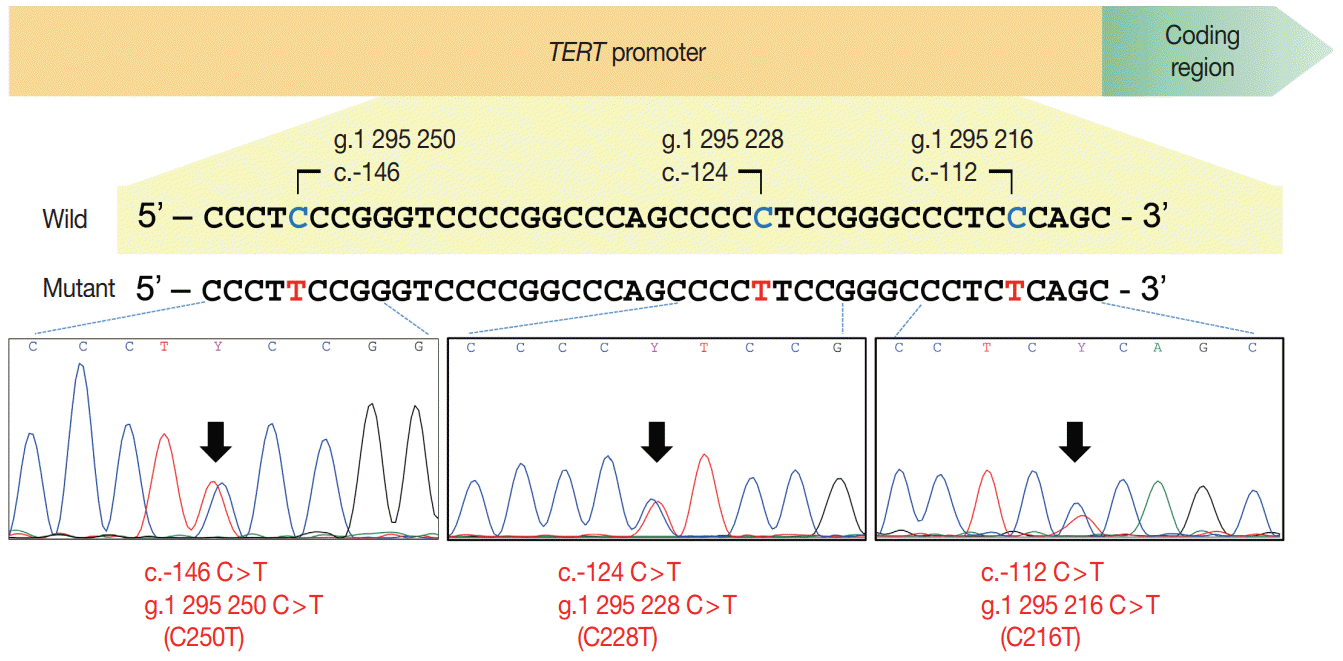 | Fig. 1.Schematic figure of the telomerase reverse transcriptase (TERT) promoter region and sequencing electropherograms of two hotspot mutations (C228T and C250T) and a C216T variant in the TERT promoter. The hotspot mutations resulted from a cytosine-to-thymine transition at genomic loci Chr5:1,295,228 (C228T) and 1,295,250 (C250T), respectively. The C216T variant is a cytosine-to-thymine transition at the 1,295,216 position of Chr5. |
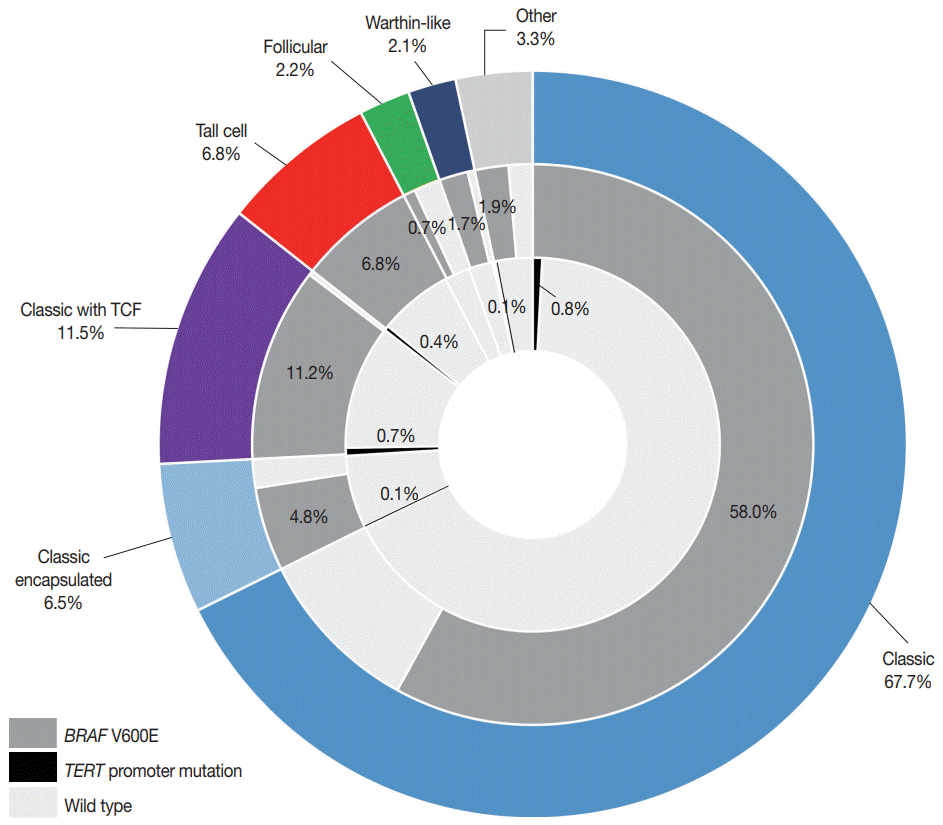 | Fig. 2.A pie chart depicting a portion of BRAF V600E and telomerase reverse transcriptase (TERT) promoter mutations (C228T and C250T) in relation to histologic variants of papillary thyroid carcinoma (n=724). The middle and inner circles show the frequency of BRAF V600E and TERT promoter mutations, respectively. The other variants included eight diffuse sclerosing, eight oncocytic, five solid, three hobnail, and one cribriform-morular variant. TCF, tall cell features. |
Table 2.
| Variable |
TERT promoter alteration, n (%) |
p-value |
||||
|---|---|---|---|---|---|---|
| Wild-type (A) | C228T, C250T (B) | C216T (C) | A vs. B | B vs. C | A vs. C | |
| Age at diagnosis (yr) | < .001 | .032 | > .99 | |||
| < 55 | 526 (99.1) | 2 (0.4) | 3 (0.6) | |||
| ≥ 55 | 178 (92.2) | 14 (7.3) | 1 (0.5) | |||
| Sex | .776 | .587 | .295 | |||
| Female | 515 (97.5) | 11 (2.1) | 2 (0.4) | |||
| Male | 189 (96.4) | 5 (2.6) | 2 (1.0) | |||
| Tumor size (cm) | .001 | .255 | > .99 | |||
| ≤ 1.0 | 510 (98.5) | 5 (1.0) | 3 (0.6) | |||
| > 1.0 | 194 (94.2) | 11 (5.3) | 1 (0.5) | |||
| Histologic variant | .313 | .214 | .405 | |||
| Classica | 603 (97.4) | 12 (1.9) | 4 (0.6) | |||
| Classic with TCF | 78 (94.0) | 5 (6.0) | 0 | |||
| Tall cell variant | 46 (93.9) | 3 (6.1) | 0 | |||
| Follicular variantb | 16 (100) | 0 | 0 | |||
| Otherc | 39 (97.5) | 1 (2.5) | 0 | |||
| Extrathyroidal extension | .032 | .162 | .645 | |||
| Absent | 274 (98.6) | 2 (0.7) | 2 (0.7) | |||
| Presentd | 430 (96.4) | 14 (3.1) | 2 (0.4) | |||
| Pathologic T category | < .001 | .267 | > .99 | |||
| pT1-2 | 667 (97.9) | 10 (1.5) | 4 (0.6) | |||
| pT3-4 | 37 (86.0) | 6 (14.0) | 0 | |||
| Pathologic N category | .297 | .619 | .322 | |||
| pN0 | 304 (96.2) | 9 (2.8) | 3 (0.9) | |||
| pN1 | 400 (98.0) | 7 (1.7) | 1 (0.2) | |||
| Lateral lymph node metastasis | .041 | > .99 | .294 | |||
| Absent | 646 (97.7) | 12 (1.8) | 3 (0.5) | |||
| Present | 58 (92.1) | 4 (6.3) | 1 (1.6) | |||
| ATA recurrence risk | < .001 | .014 | .344 | |||
| Low risk | 237 (98.3) | 2 (0.8) | 2 (0.8) | |||
| Intermediate risk | 354 (98.9) | 2 (0.6) | 2 (0.6) | |||
| High risk | 113 (90.4) | 12 (9.6) | 0 | |||
| AJCC cancer staging, 8th edition | .065 | > .99 | > .99 | |||
| Stage I/II | 702 (97.4) | 15 (2.1) | 4 (0.6) | |||
| Stage III/IV | 2 (66.7) | 1 (33.3) | 0 | |||
| BRAF V600E mutation | .489 | > .99 | > .99 | |||
| Absent | 107 (99.1) | 1 (0.9) | 0 | |||
| Present | 597 (96.9) | 15 (2.4) | 4 (0.6) | |||
TERT, telomerase reverse transcriptase; TCF, tall cell features; ATA, American Thyroid Association; AJCC, American Joint Committee on Cancer.
a Classic papillary thyroid carcinoma (PTC) included classic PTC (n = 490), classic PTC with tall cell features (n = 83) and encapsulated classic PTC (n = 46);
b Follicular variant included infiltrative follicular variant (n = 10) and invasive encapsulated follicular variant (n = 6);




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download