Abstract
Notes
Ethics Statement
This study was approved by the Institutional Review Board of Ulsan University Hospital with a waiver of informed consent (IRB No. 2019-12-002).
Author contributions
Conceptualization: HJC (Hee Jeong Cha), KK.
Investigation: HJC (Hee Jeong Cha), KK, SC.
Supervision: HJC (Hee Jeong Cha).
Visualization: HJC (Hee Jeong Cha), KK, MJS.
Writing—original draft: HJC (Hee Jeong Cha), KK, SC.
Writing—review & editing: JHS, YMK, HJC (Hye Jeong Choi), HJC (Hee Jeong Cha), KK, MK, MJS, JHK, SC.
REFERENCES
Fig. 1.
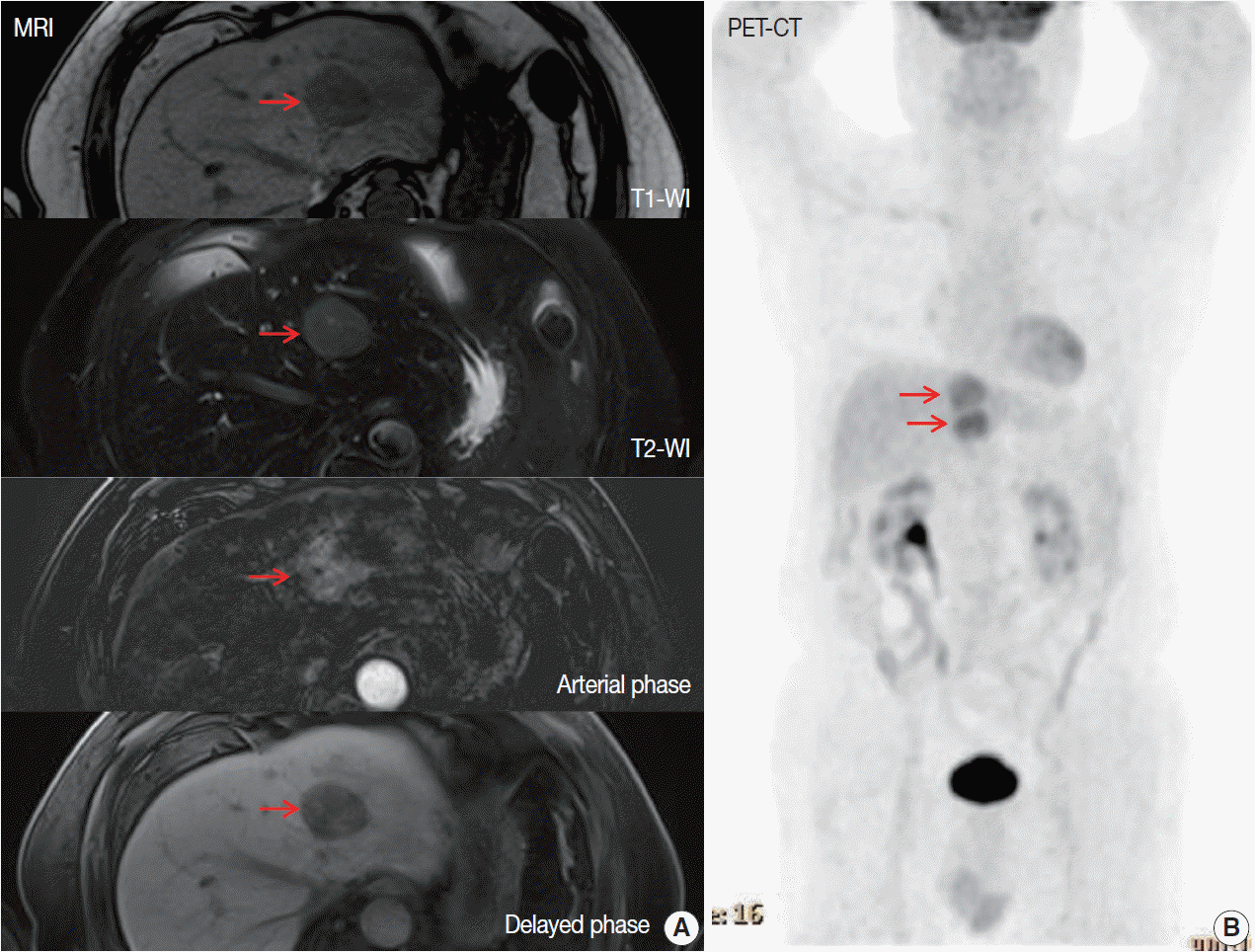
Fig. 2.
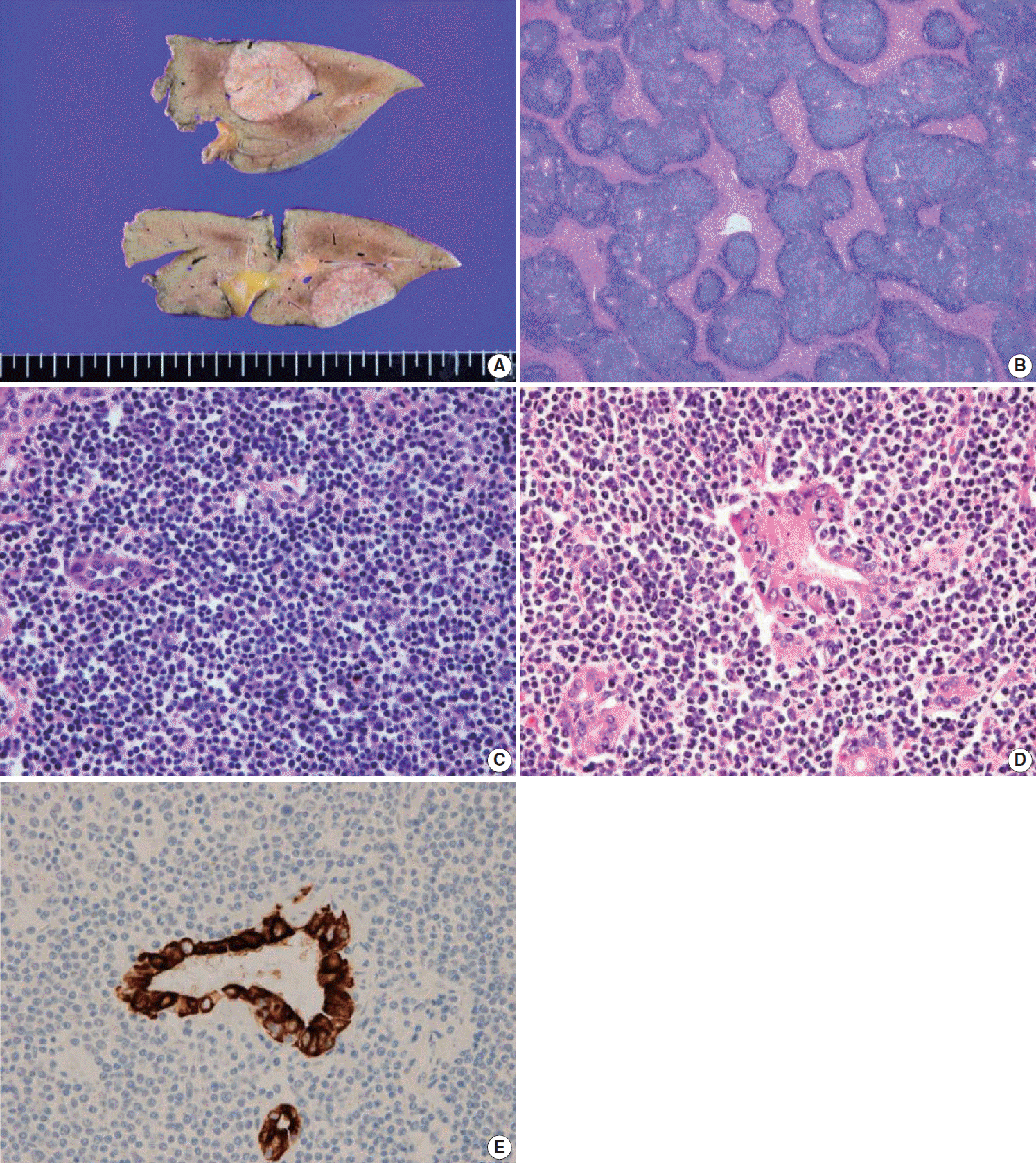
Fig. 3.
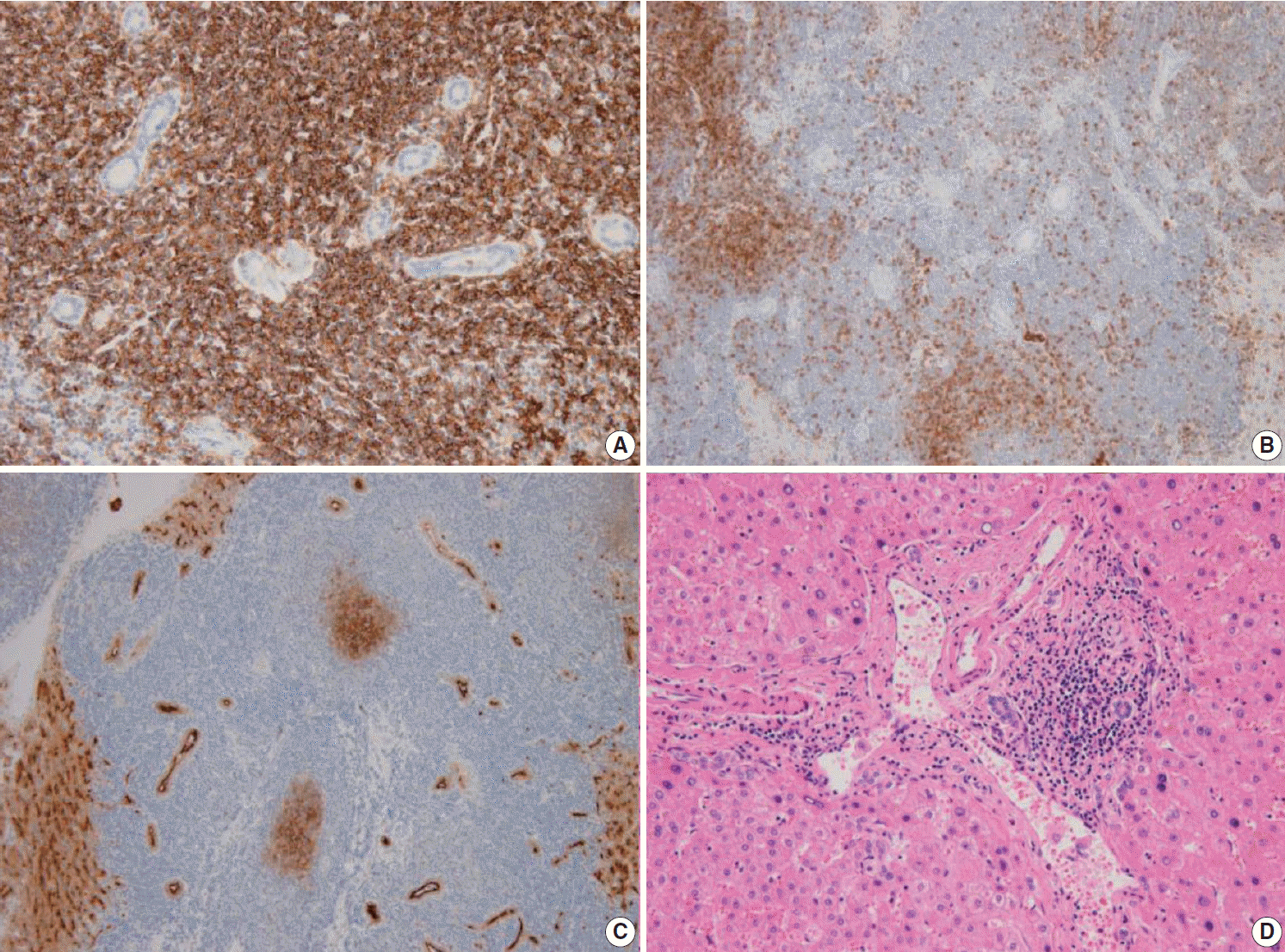
Table 1.
| Case No. | Study | Age (yr)/Sex | HBV | HCV | Comorbidities | Image finding | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | Xie et al. [6] | 73/M | (+) | (–) | None | CT: enhanced in arterial phase | Surgery | Alive/6 mo |
| MRI: hypointense on T1, hyperintense on T2 | ||||||||
| 2 | Nagata et al. [7] | 74/M | (–) | (–) | Hypertension | CT: no mass | Surgery | Alive/2 yr |
| MRI: enhanced on T1 in arterial phase, withdrawal in portal venous and delayed phases | ||||||||
| 3 | Betianu et al. [8] | 47/F | (–) | (–) | None | CT: hypodense mass | Surgery and chemotherapy | Alive/9 mo |
| MRI: hypointense on T1, hyperintense on T2 | ||||||||
| 4 | Bao et al. [9] | 59/F | (+) | (–) | None | CT: hypodense lesions | Patient refused | ND |
| PET/CT: increased uptake of SUV | ||||||||
| 5 | Obiorah et al. [10] | 80/F | (–) | (–) | None | CT: bilobed mass | Surgery, rituximab and idelalisib | Pulmonary recur/1 yr, parotid gland recur/7 yr |
| 6 | Obiorah et al. [10] | 30/F | (–) | (–) | Autoimmune hepatitis, Hashimoto’s thyroiditis | PET/CT: diffuse FDG activity | Rituximab | Alive/3 yr |
| 7 | Bohlok et al. [11] | 68/M | (–) | (–) | Alcoholic cirrhosis, hemochromatosis | MRI: enhanced in arterial phase and washout in portal phase | Surgery | ND |
| FDG PET: hypermetabolic tumor | ||||||||
| 8 | Khurana et al. [12] | 71/F | (–) | (–) | Invasive breast cancer, papillary thyroid carcinoma | PET: FDG hypodense lesion | Surgery | ND |
| 9 | Dong et al. [13] | 50/M | (–) | (–) | Type 2 diabetes, hypertension | MRI: hypointense on T1 and hyperintense on T2, enhanced in the arterial phase and portal phase | Surgery and Rituximab | Alive/13 mo |
| 10 | Li et al. [14] | 44/F | (–) | (+) | None | MRI: hyperintense on T1 and T2 | Surgery | Alive/27 mo |
| 11 | Haefliger et al. [15] | 69/M | (–) | (–) | Non-alcoholic steatohepatitis, Helicobacter pylori–associated gastritis | MRI: hyperintense on T2, washout on portal venous phase | Rituximab and HP eradication therapy | Alive/6 mo |
| 12 | Our case | 70/M | (–) | (–) | Intracerebral hemorrhage, myocardial infarction | MRI: enhanced in arterial phase | Surgery | Alive/8 mo |
| PET/CT: hypermetabolic and isometabolic nodules |
MALT, mucosa-associated lymphoid tissue; HBV, hepatitis B virus; HCV, hepatitis C virus; M, male; CT, computed tomography; MRI, magnetic resonance imaging; F, female; PET, positron emission tomography; SUV, standardized uptake value; ND, not detected; FDG, fluorodeoxyglucose; HP, Helicobacter pylori.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download