1. Harris NL, Swerdlow SH, Jaffe ES, et al. Follicular lymphoma. In : Swerdlow SH, Campo E, Harris NL, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC Press;2008. p. 220–6.
2. Jung HR, Huh J, Ko YH, et al. Classification of malignant lymphoma subtypes in Korean patients: a report of the 4th nationwide study. J Hematopathol. 2019; 12:173–81.

3. Goodlad JR, Batstone PJ, Hamilton D, Hollowood K. Follicular lymphoma with marginal zone differentiation: cytogenetic findings in support of a high-risk variant of follicular lymphoma. Histopathology. 2003; 42:292–8.

4. Goates JJ, Kamel OW, LeBrun DP, Benharroch D, Dorfman RF. Floral variant of follicular lymphoma. Immunological and molecular studies support a neoplastic process. Am J Surg Pathol. 1994; 18:37–47.
5. Kojima M, Matsumoto M, Miyazawa Y, Shimizu K, Itoh H, Masawa N. Follicular lymphoma with prominent sclerosis (“sclerosing variant of follicular lymphoma”) exhibiting a mesenteric bulky mass resembling inflammatory pseudotumor: report of three cases. Pathol Oncol Res. 2007; 13:74–7.

6. Kim JM, Ko YH, Lee SS, et al. WHO classification of malignant lymphomas in Korea: report of the third nationwide study. Korean J Pathol. 2011; 45:254–60.

7. Warnke RA, Weiss LM, Chan JK, Cleary ML, Dorfman RF. Tumors of the lymph nodes and spleen. In : Rosai J, Sobin LH, editors. Atlans of tumor pathology. 3rd series. Washington, DC: Armed Forces Institute of Pathology;1994. p. 85.
8. Nozawa Y, Hirao M, Kamimura K, Hara Y, Abe M. Unusual case of follicular lymphoma with hyaline-vascular follicles. Pathol Int. 2002; 52:794–5.

9. Kojima M, Sakurai S, Isoda A, Tsukamoto N, Masawa N, Nakamura N. Follicular lymphoma resembling with hyaline-vascular type of Castleman’s disease: the morphological and immunohistochemical findings of two cases. Cancer Ther. 2009; 7:109–12.
10. Siddiqi IN, Brynes RK, Wang E. B-cell lymphoma with hyaline vascular Castleman disease-like features: a clinicopathologic study. Am J Clin Pathol. 2011; 135:901–14.
11. Pina-Oviedo S, Wang W, Vicknair E, Manning JT Jr, Medeiros LJ. Follicular lymphoma with hyaline-vascular Castleman disease-like follicles and CD20 positive follicular dendritic cells. Pathology. 2017; 49:544–7.

12. Pina-Oviedo S, Miranda RN, Lin P, Manning JT, Medeiros LJ. Follicular lymphoma with hyaline-vascular Castleman-like features: analysis of 6 cases and review of the literature. Hum Pathol. 2017; 68:136–46.

13. Kagami Y, Jung J, Choi YS, et al. Establishment of a follicular lymphoma cell line (FLK-1) dependent on follicular dendritic cell-like cell line HK. Leukemia. 2001; 15:148–56.

14. Takata K, Sato Y, Nakamura N, et al. Duodenal and nodal follicular lymphomas are distinct: the former lacks activation-induced cytidine deaminase and follicular dendritic cells despite ongoing somatic hypermutations. Mod Pathol. 2009; 22:940–9.

15. Chang KC, Wang YC, Hung LY, et al. Monoclonality and cytogenetic abnormalities in hyaline vascular Castleman disease. Mod Pathol. 2014; 27:823–31.

16. Freedman A. Follicular lymphoma: 2015 update on diagnosis and management. Am J Hematol. 2015; 90:1171–8.

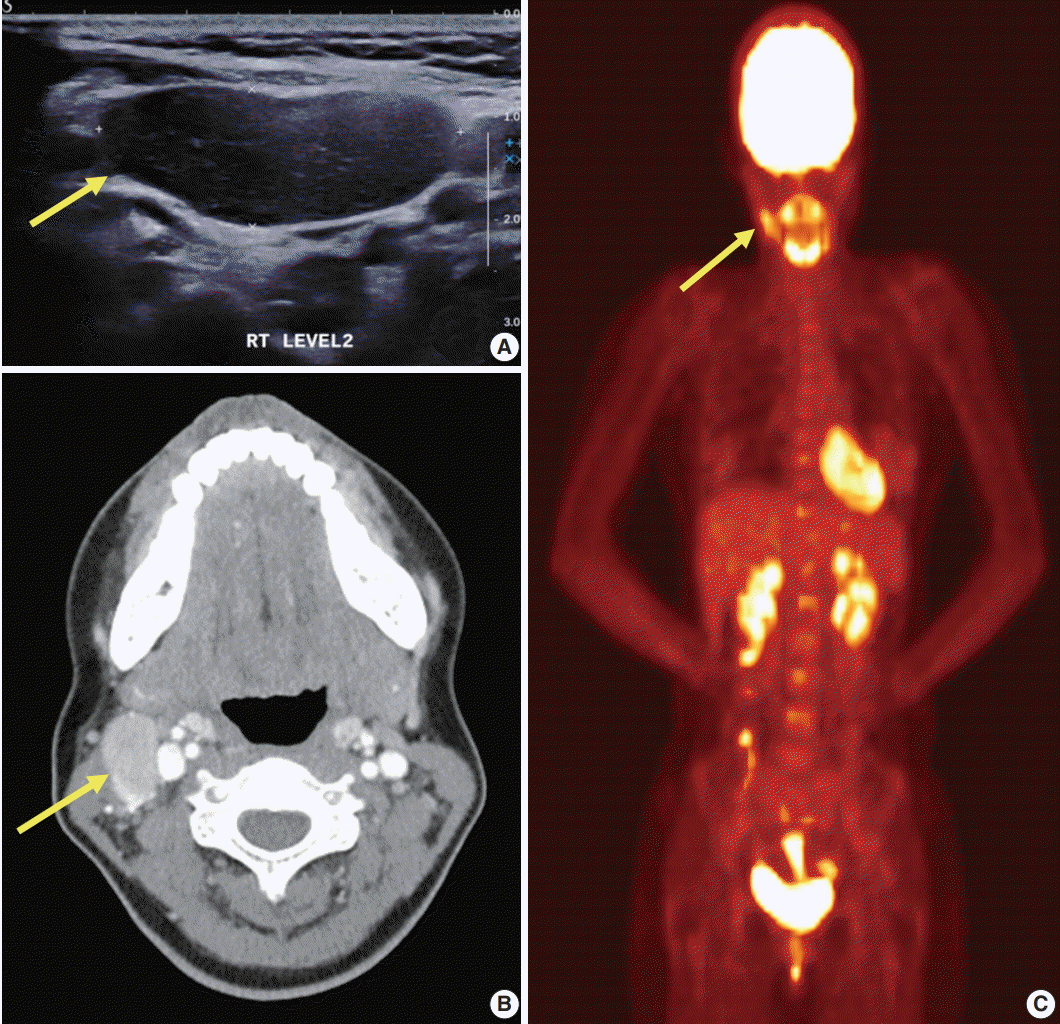

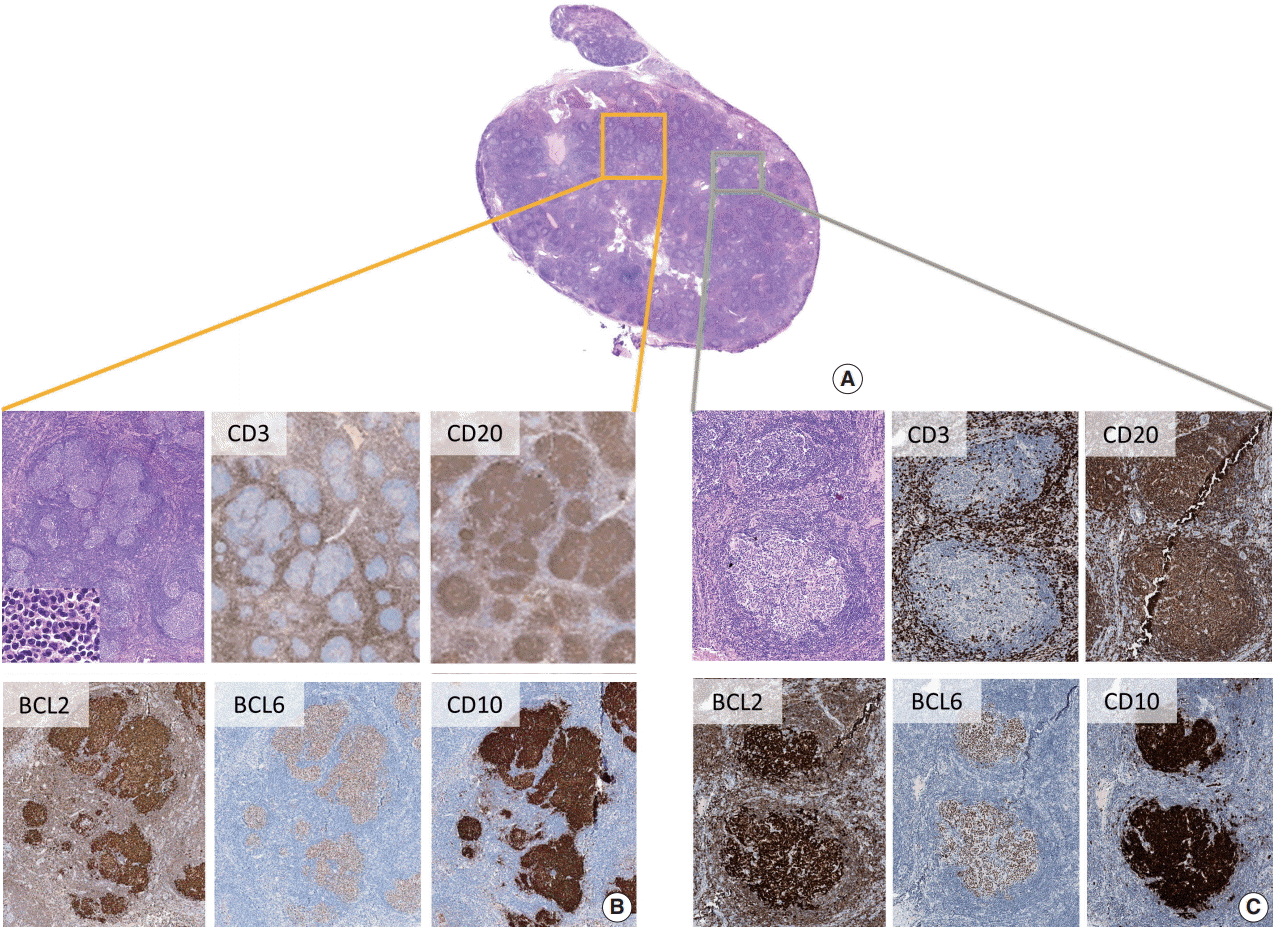




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download