1. Pitman MB, Abele J, Ali SZ, et al. Techniques for thyroid FNA: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008; 36:407–24.

2. Ha EJ, Lim HK, Yoon JH, et al. Primary imaging test and appropriate biopsy methods for thyroid nodules: guidelines by Korean Society of Radiology and National Evidence-Based Healthcare Collaborating Agency. Korean J Radiol. 2018; 19:623–31.

3. Ashcraft MW, Van Herle AJ. Management of thyroid nodules. II: Scanning techniques, thyroid suppressive therapy, and fine needle aspiration. Head Neck Surg. 1981; 3:297–322.

4. Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW. The Bethesda System for Reporting Thyroid Cytopathology: a meta-analysis. Acta Cytol. 2012; 56:333–9.

5. Alexander EK, Heering JP, Benson CB, et al. Assessment of non-diagnostic ultrasound-guided fine needle aspirations of thyroid nodules. J Clin Endocrinol Metab. 2002; 87:4924–7.

6. Orija IB, Pineyro M, Biscotti C, Reddy SS, Hamrahian AH. Value of repeating a non-diagnostic thyroid fine-needle aspiration biopsy. Endocr Pract. 2007; 13:735–42.

7. Nayar R, Ivanovic M. The indeterminate thyroid fine-needle aspiration: experience from an academic center using terminology similar to that proposed in the 2007 National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Cancer. 2009; 117:195–202.
8. Yang J, Schnadig V, Logrono R, Wasserman PG. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer. 2007; 111:306–15.

9. Yassa L, Cibas ES, Benson CB, et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer. 2007; 111:508–16.

10. Bongiovanni M, Krane JF, Cibas ES, Faquin WC. The atypical thyroid fine-needle aspiration: past, present, and future. Cancer Cytopathol. 2012; 120:73–86.

11. Deveci MS, Deveci G, LiVolsi VA, Baloch ZW. Fine-needle aspiration of follicular lesions of the thyroid: diagnosis and follow-up. Cytojournal. 2006; 3:9.
12. Yoo C, Choi HJ, Im S, et al. Fine needle aspiration cytology of thyroid follicular neoplasm: cytohistologic correlation and accuracy. Korean J Pathol. 2013; 47:61–6.

13. Novoa E, Gurtler N, Arnoux A, Kraft M. Role of ultrasound-guided core-needle biopsy in the assessment of head and neck lesions: a meta-analysis and systematic review of the literature. Head Neck. 2012; 34:1497–503.

14. Na DG, Baek JH, Jung SL, et al. Core needle biopsy of the thyroid: 2016 consensus statement and recommendations from Korean Society of Thyroid Radiology. Korean J Radiol. 2017; 18:217–37.

15. Ha EJ, Baek JH, Lee JH, et al. Complications following US-guided core-needle biopsy for thyroid lesions: a retrospective study of 6,169 consecutive patients with 6,687 thyroid nodules. Eur Radiol. 2017; 27:1186–94.

16. Paja M, Del Cura JL, Zabala R, Korta I, Ugalde A, Lopez JI. Coreneedle biopsy in thyroid nodules: performance, accuracy, and complications. Eur Radiol. 2019; 29:4889–96.

17. Ha EJ, Suh CH, Baek JH. Complications following ultrasoundguided core needle biopsy of thyroid nodules: a systematic review and meta-analysis. Eur Radiol. 2018; 28:3848–60.

18. Na DG, Kim JH, Sung JY, et al. Core-needle biopsy is more useful than repeat fine-needle aspiration in thyroid nodules read as non-diagnostic or atypia of undetermined significance by the Bethesda system for reporting thyroid cytopathology. Thyroid. 2012; 22:468–75.

19. Samir AE, Vij A, Seale MK, et al. Ultrasound-guided percutaneous thyroid nodule core biopsy: clinical utility in patients with prior non-diagnostic fine-needle aspirate. Thyroid. 2012; 22:461–7.

20. Cao H, Kao RH, Hsieh MC. Comparison of core-needle biopsy and fine-needle aspiration in screening for thyroid malignancy: a systematic review and meta-analysis. Curr Med Res Opin. 2016; 32:1291–301.

21. Pyo JS, Sohn JH, Kang G. Core needle biopsy is a more conclusive follow-up method than repeat fine needle aspiration for thyroid nodules with initially inconclusive results: a systematic review and meta-analysis. J Pathol Transl Med. 2016; 50:217–24.

22. Trimboli P, Nasrollah N, Guidobaldi L, et al. The use of core needle biopsy as first-line in diagnosis of thyroid nodules reduces false negative and inconclusive data reported by fine-needle aspiration. World J Surg Oncol. 2014; 12:61.

23. Suh CH, Baek JH, Choi YJ, et al. Efficacy and safety of core-needle biopsy in initially detected thyroid nodules via propensity score analysis. Sci Rep. 2017; 7:8242.

24. Kim HC, Kim YJ, Han HY, et al. First-line use of core needle biopsy for high-yield preliminary diagnosis of thyroid nodules. AJNR Am J Neuroradiol. 2017; 38:357–63.

25. Hong MJ, Na DG, Kim SJ, Kim DS. Role of core needle biopsy as a first-line diagnostic tool for thyroid nodules: a retrospective cohort study. Ultrasonography. 2018; 37:244–53.

26. Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2017; 27:1341–6.

27. Baek JH, Na DG, Lee JH, et al. Core needle biopsy of thyroid nodules: consensus statement and recommendations. J Korean Soc Ultrasound Med. 2013; 32:95–102.
28. Jung CK, Min HS, Park HJ, et al. Pathology reporting of thyroid core needle biopsy: a proposal of the Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group. J Pathol Transl Med. 2015; 49:288–99.

29. Lloyd RV, Osamura RY, Klöppel G, Rosai J. WHO classification of tumours of endocrine organs. 4th ed. Lyon: IARC Press;2017. p. 66–103.
30. Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: 2016 update. Endocr Pract. 2016; 22:622–39.
31. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; 26:1–133.
32. Baloch ZW, Cibas ES, Clark DP, et al. The National Cancer Institute Thyroid fine needle aspiration state of the science conference: a summation. Cytojournal. 2008; 5:6.

33. Perros P, Boelaert K, Colley S, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf). 2014; 81 Suppl 1:1–122.

34. Yim Y, Baek JH. Core needle biopsy in the management of thyroid nodules with an indeterminate fine-needle aspiration report. Gland Surg. 2019; 8(Suppl 2):S77–S85.

35. Jung CK, Baek JH. Recent advances in core needle biopsy for thyroid nodules. Endocrinol Metab (Seoul). 2017; 32:407–12.

36. Suh CH, Baek JH, Kim KW, et al. The role of core-needle biopsy for thyroid nodules with initially non-diagnostic fine-needle aspiration results: a systematic review and meta-analysis. Endocr Pract. 2016; 22:679–88.

37. Paja M, del Cura JL, Zabala R, et al. Ultrasound-guided core-needle biopsy in thyroid nodules: a study of 676 consecutive cases with surgical correlation. Eur Radiol. 2016; 26:1–8.

38. Suh CH, Baek JH, Lee JH, et al. The role of core-needle biopsy as a first-line diagnostic tool for initially detected thyroid nodules. Thyroid. 2016; 26:395–403.

39. Sung JY, Na DG, Kim KS, et al. Diagnostic accuracy of fine-needle aspiration versus core-needle biopsy for the diagnosis of thyroid malignancy in a clinical cohort. Eur Radiol. 2012; 22:1564–72.

40. Chung SR, Suh CH, Baek JH, Choi YJ, Lee JH. The role of core needle biopsy in the diagnosis of initially detected thyroid nodules: a systematic review and meta-analysis. Eur Radiol. 2018; 28:4909–18.

41. Screaton NJ, Berman LH, Grant JW. US-guided core-needle biopsy of the thyroid gland. Radiology. 2003; 226:827–32.

42. Yeon JS, Baek JH, Lim HK, et al. Thyroid nodules with initially non-diagnostic cytologic results: the role of core-needle biopsy. Radiology. 2013; 268:274–80.

43. Park KT, Ahn SH, Mo JH, et al. Role of core needle biopsy and ultrasonographic finding in management of indeterminate thyroid nodules. Head Neck. 2011; 33:160–5.

44. Lee KH, Shin JH, Oh YL, Hahn SY. Atypia of undetermined significance in thyroid fine-needle aspiration cytology: prediction of malignancy by US and comparison of methods for further management. Ann Surg Oncol. 2014; 21:2326–31.

45. Na DG, Min HS, Lee H, Won JK, Seo HB, Kim JH. Role of core needle biopsy in the management of atypia/follicular lesion of undetermined significance thyroid nodules: comparison with repeat fine-needle aspiration in subcategory nodules. Eur Thyroid J. 2015; 4:189–96.

46. Buxey K, Serpell J. Importance of core biopsy in the diagnosis of thyroid lymphoma. ANZ J Surg. 2012; 82:90.

47. Kwak JY, Kim EK, Ko KH, et al. Primary thyroid lymphoma: role of ultrasound-guided needle biopsy. J Ultrasound Med. 2007; 26:1761–5.
48. Nam M, Shin JH, Han BK, et al. Thyroid lymphoma: correlation of radiologic and pathologic features. J Ultrasound Med. 2012; 31:589–94.
49. Ha EJ, Baek JH, Na DG, et al. The role of core needle biopsy and its impact on surgical management in patients with medullary thyroid cancer: clinical experience at 3 medical institutions. AJNR Am J Neuroradiol. 2015; 36:1512–7.

50. Kwok A, Faigel DO. Management of anticoagulation before and after gastrointestinal endoscopy. Am J Gastroenterol. 2009; 104:3085–97.

51. Baek JH. Current status of core needle biopsy of the thyroid. Ultrasonography. 2017; 36:83–5.

52. Del Cura JL, Zabala R, Corta I. US-guided interventional procedures: what a radiologist needs to know. Radiologia. 2010; 52:198–207.

53. Harvey JN, Parker D, De P, Shrimali RK, Otter M. Sonographically guided core biopsy in the assessment of thyroid nodules. J Clin Ultrasound. 2005; 33:57–62.

54. Nasrollah N, Trimboli P, Guidobaldi L, et al. Thin core biopsy should help to discriminate thyroid nodules cytologically classified as indeterminate: a new sampling technique. Endocrine. 2013; 43:659–65.

55. Zhang M, Zhang Y, Fu S, Lv F, Tang J. Thyroid nodules with suspicious ultrasound findings: the role of ultrasound-guided core needle biopsy. Clin Imaging. 2014; 38:434–8.

56. Hahn SY, Shin JH, Han BK, Ko EY, Ko ES. Ultrasonography-guided core needle biopsy for the thyroid nodule: does the procedure hold any benefit for the diagnosis when fine-needle aspiration cytology analysis shows inconclusive results? Br J Radiol. 2013; 86:20130007.

57. Min HS, Kim JH, Ryoo I, Jung SL, Jung CK. The role of core needle biopsy in the preoperative diagnosis of follicular neoplasm of the thyroid. APMIS. 2014; 122:993–1000.

58. Yoon RG, Baek JH, Lee JH, et al. Diagnosis of thyroid follicular neoplasm: fine-needle aspiration versus core-needle biopsy. Thyroid. 2014; 24:1612–7.

59. Ahn HS, Seo M, Ha SM, Kim HS. Comparison of the diagnostic efficacy of ultrasound-guided core needle biopsy with 18- versus 20-gauge needles for thyroid nodules. J Ultrasound Med. 2018; 37:2565–74.

60. Lopez JI, Zabala R, Del Cura JL. Histological diagnosis of thyroid disease using ultrasound-guided core biopsies. Eur Thyroid J. 2013; 2:29–36.

61. Hahn SY, Shin JH, Oh YL. What is the ideal core number for ultrasonography-guided thyroid biopsy of cytologically inconclusive nodules? AJNR Am J Neuroradiol. 2017; 38:777–81.

62. Park HS, Baek JH, Gyu ND. Regarding "what is the ideal core number for ultrasonography-guided thyroid biopsy of cytologically inconclusive nodules?". AJNR Am J Neuroradiol. 2017; 38:E53–4.

63. Chung SR, Baek JH, Choi YJ, et al. The role of core needle biopsy for the evaluation of thyroid nodules with suspicious ultrasound features. Korean J Radiol. 2019; 20:158–65.

64. Hong MJ, Sung JY, Baek JH, et al. Safety and efficacy of radiofrequency ablation for nonfunctioning benign thyroid nodules in children and adolescents in 14 patients over a 10-year period. J Vasc Interv Radiol. 2019; 30:900–6.

65. Chung SR, Baek JH, Lee JH, et al. Risk of malignancy according to the sub-classification of atypia of undetermined significance and suspicious follicular neoplasm categories in thyroid core needle biopsies. Endocr Pathol. 2019; 30:146–54.

66. Ha EJ, Baek JH, Lee JH, et al. Core needle biopsy can minimise the non-diagnostic results and need for diagnostic surgery in patients with calcified thyroid nodules. Eur Radiol. 2014; 24:1403–9.

67. Yi KS, Kim JH, Na DG, et al. Usefulness of core needle biopsy for thyroid nodules with macrocalcifications: comparison with fine-needle aspiration. Thyroid. 2015; 25:657–64.

68. Na DG, Kim DS, Kim SJ, Ryoo JW, Jung SL. Thyroid nodules with isolated macrocalcification: malignancy risk and diagnostic efficacy of fine-needle aspiration and core needle biopsy. Ultrasonography. 2016; 35:212–9.

69. Ren J, Baek JH, Chung SR, Choi YJ, Jung CK, Lee JH. Degenerating thyroid nodules: ultrasound diagnosis, clinical significance, and management. Korean J Radiol. 2019; 20:947–55.

70. Khoo TK, Baker CH, Hallanger-Johnson J, et al. Comparison of ultrasound-guided fine-needle aspiration biopsy with core-needle biopsy in the evaluation of thyroid nodules. Endocr Pract. 2008; 14:426–31.

71. Quinn SF, Nelson HA, Demlow TA. Thyroid biopsies: fine-needle aspiration biopsy versus spring-activated core biopsy needle in 102 patients. J Vasc Interv Radiol. 1994; 5:619–23.

72. Ha EJ, Baek JH, Lee JH. Ultrasonography-based thyroidal and perithyroidal anatomy and its clinical significance. Korean J Radiol. 2015; 16:749–66.

73. Nasrollah N, Trimboli P, Rossi F, et al. Patient's comfort with and tolerability of thyroid core needle biopsy. Endocrine. 2014; 45:79–83.

74. Jeong EJ, Chung SR, Baek JH, Choi YJ, Kim JK, Lee JH. A comparison of ultrasound-guided fine needle aspiration versus core needle biopsy for thyroid nodules: pain, tolerability, and complications. Endocrinol Metab (Seoul). 2018; 33:114–20.

75. Kim HJ, Kim YK, Moon JH, Choi JY, Choi SI. Thyroid core needle biopsy: patients' pain and satisfaction compared to fine needle aspiration. Endocrine. 2019; 65:365–70.

76. Karstrup S, Balslev E, Juul N, Eskildsen PC, Baumbach L. US-guided fine needle aspiration versus coarse needle biopsy of thyroid nodules. Eur J Ultrasound. 2001; 13:1–5.

77. Polyzos SA, Anastasilakis AD. Clinical complications following thyroid fine-needle biopsy: a systematic review. Clin Endocrinol (Oxf). 2009; 71:157–65.

78. Kakiuchi Y, Idota N, Nakamura M, Ikegaya H. A fatal case of cervical hemorrhage after fine needle aspiration and core needle biopsy of the thyroid gland. Am J Forensic Med Pathol. 2015; 36:207–9.

79. Tomoda C, Takamura Y, Ito Y, Miya A, Miyauchi A. Transient vocal cord paralysis after fine-needle aspiration biopsy of thyroid tumor. Thyroid. 2006; 16:697–9.

80. Choi SH, Kim EK, Kim SJ, Kwak JY. Thyroid ultrasonography: pitfalls and techniques. Korean J Radiol. 2014; 15:267–76.

81. Bergeron M, Beaudoin D. Simple core-needle biopsy for thyroid nodule, complicated tinnitus. Eur Thyroid J. 2014; 3:130–3.

82. Shah KS, Ethunandan M. Tumour seeding after fine-needle aspiration and core biopsy of the head and neck: a systematic review. Br J Oral Maxillofac Surg. 2016; 54:260–5.
83. Thavarajah R, Mudimbaimannar VK, Elizabeth J, Rao UK, Ranganathan K. Chemical and physical basics of routine formaldehyde fixation. J Oral Maxillofac Pathol. 2012; 16:400–5.

84. Hewitt SM, Lewis FA, Cao Y, et al. Tissue handling and specimen preparation in surgical pathology: issues concerning the recovery of nucleic acids from formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med. 2008; 132:1929–35.

85. Xiong Y, Yan L, Nong L, Zheng Y, Li T. Pathological diagnosis of thyroid nodules based on core needle biopsies: comparative study between core needle biopsies and resected specimens in 578 cases. Diagn Pathol. 2019; 14:10.

86. Cibas ES, Ali SZ; NCI Thyroid FNA State of the Science Conference. The Bethesda System For Reporting Thyroid Cytopathology. Am J Clin Pathol. 2009; 132:658–65.

87. Ali SZ. Thyroid cytopathology: Bethesda and beyond. Acta Cytol. 2011; 55:4–12.

88. Na HY, Woo JW, Moon JH, et al. Preoperative diagnostic categories of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in thyroid core needle biopsy and its impact on risk of malignancy. Endocr Pathol. 2019; 30:329–39.

89. Bae JS, Choi SK, Jeon S, et al. Impact of NRAS mutations on the diagnosis of follicular neoplasm of the thyroid. Int J Endocrinol. 2014; 2014:289834.

90. Ustun B, Chhieng D, Van Dyke A, et al. Risk stratification in follicular neoplasm: a cytological assessment using the modified Bethesda classification. Cancer Cytopathol. 2014; 122:536–45.

91. Crescenzi A, Guidobaldi L, Nasrollah N, et al. Immunohistochemistry for BRAF(V600E) antibody VE1 performed in core needle biopsy samples identifies mutated papillary thyroid cancers. Horm Metab Res. 2014; 46:370–4.

92. Alshenawy HA. Utility of immunohistochemical markers in diagnosis of follicular cell derived thyroid lesions. Pathol Oncol Res. 2014; 20:819–28.

93. El Demellawy D, Nasr AL, Babay S, Alowami S. Diagnostic utility of CD56 immunohistochemistry in papillary carcinoma of the thyroid. Pathol Res Pract. 2009; 205:303–9.

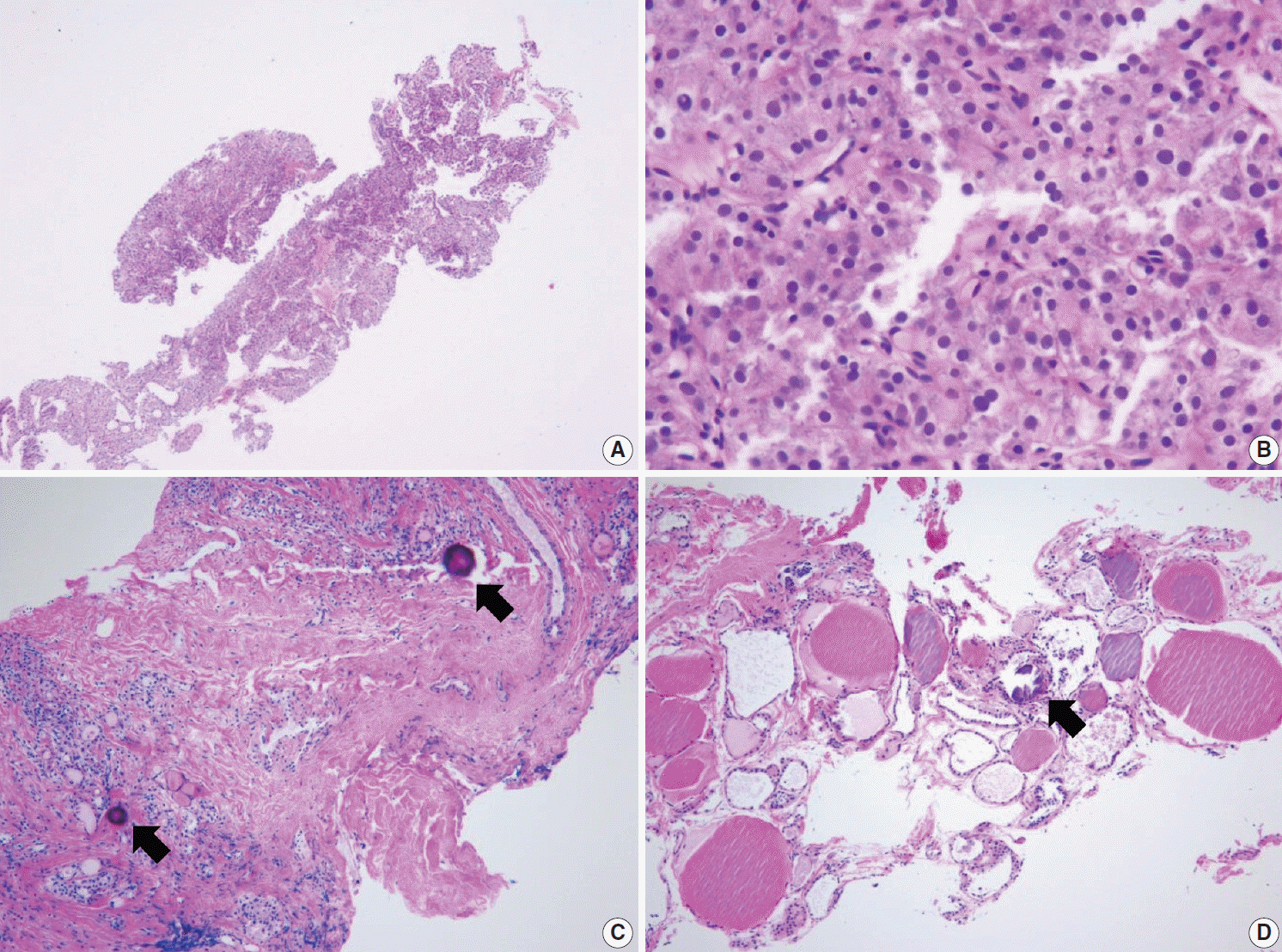
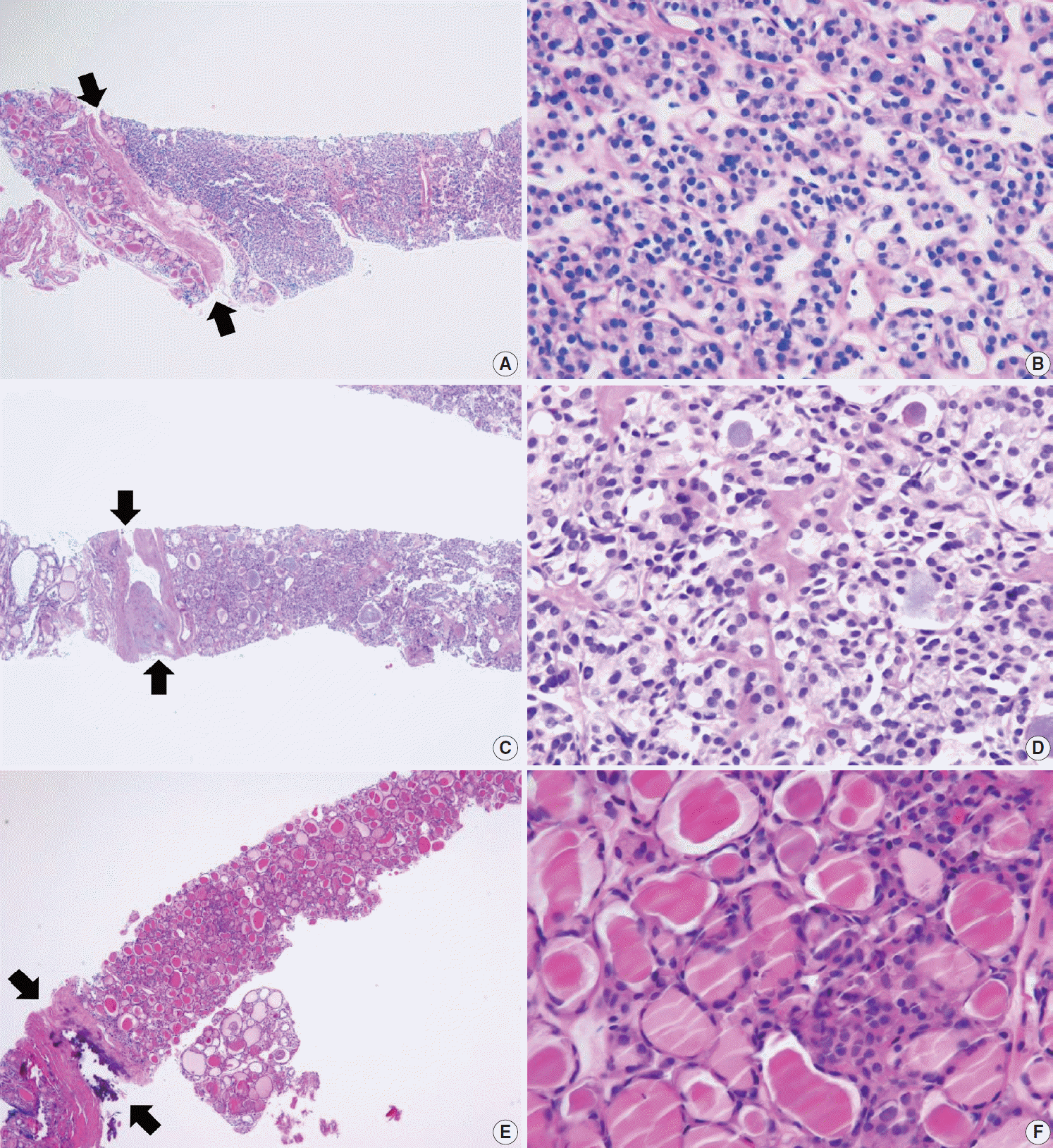
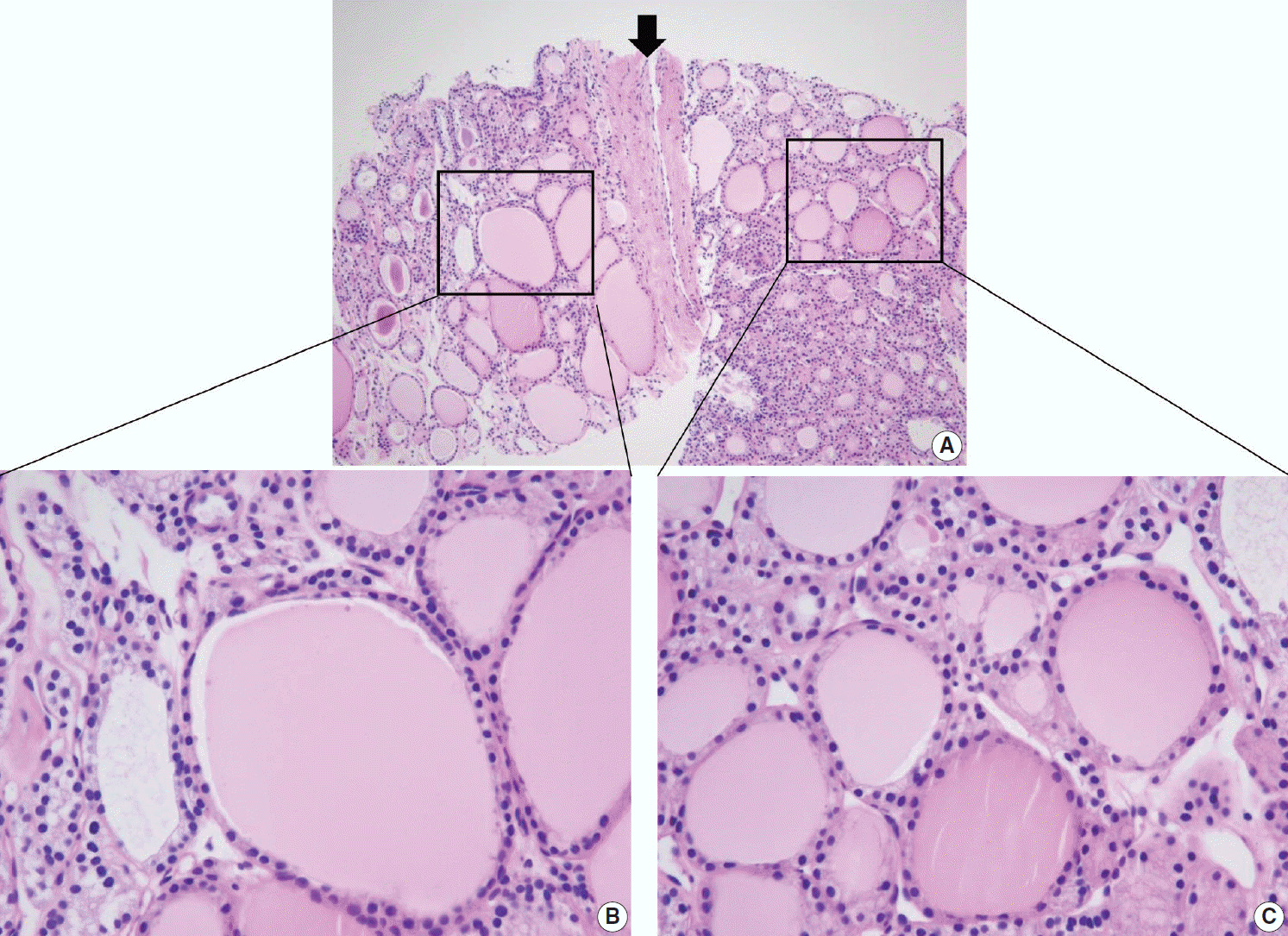
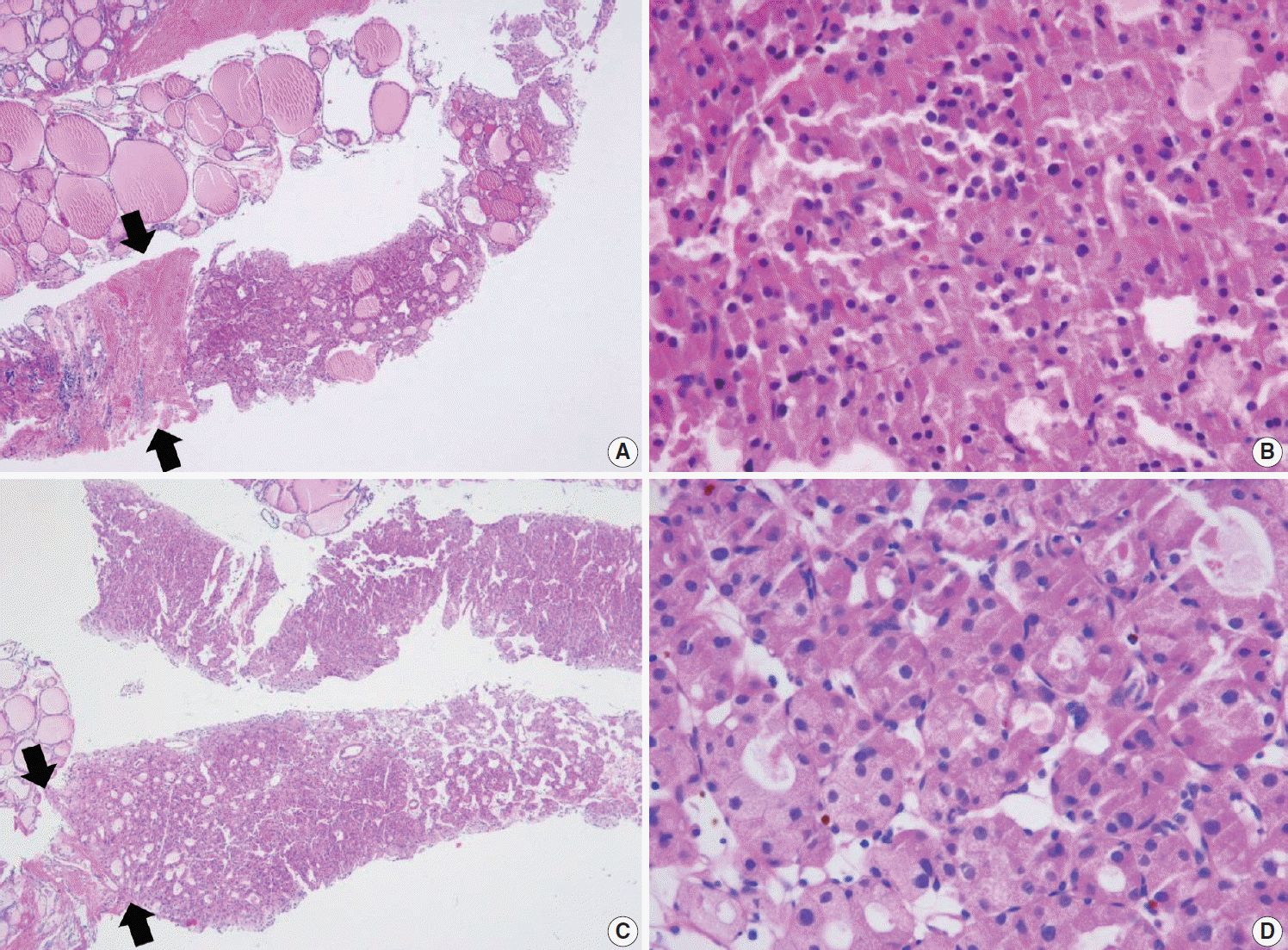
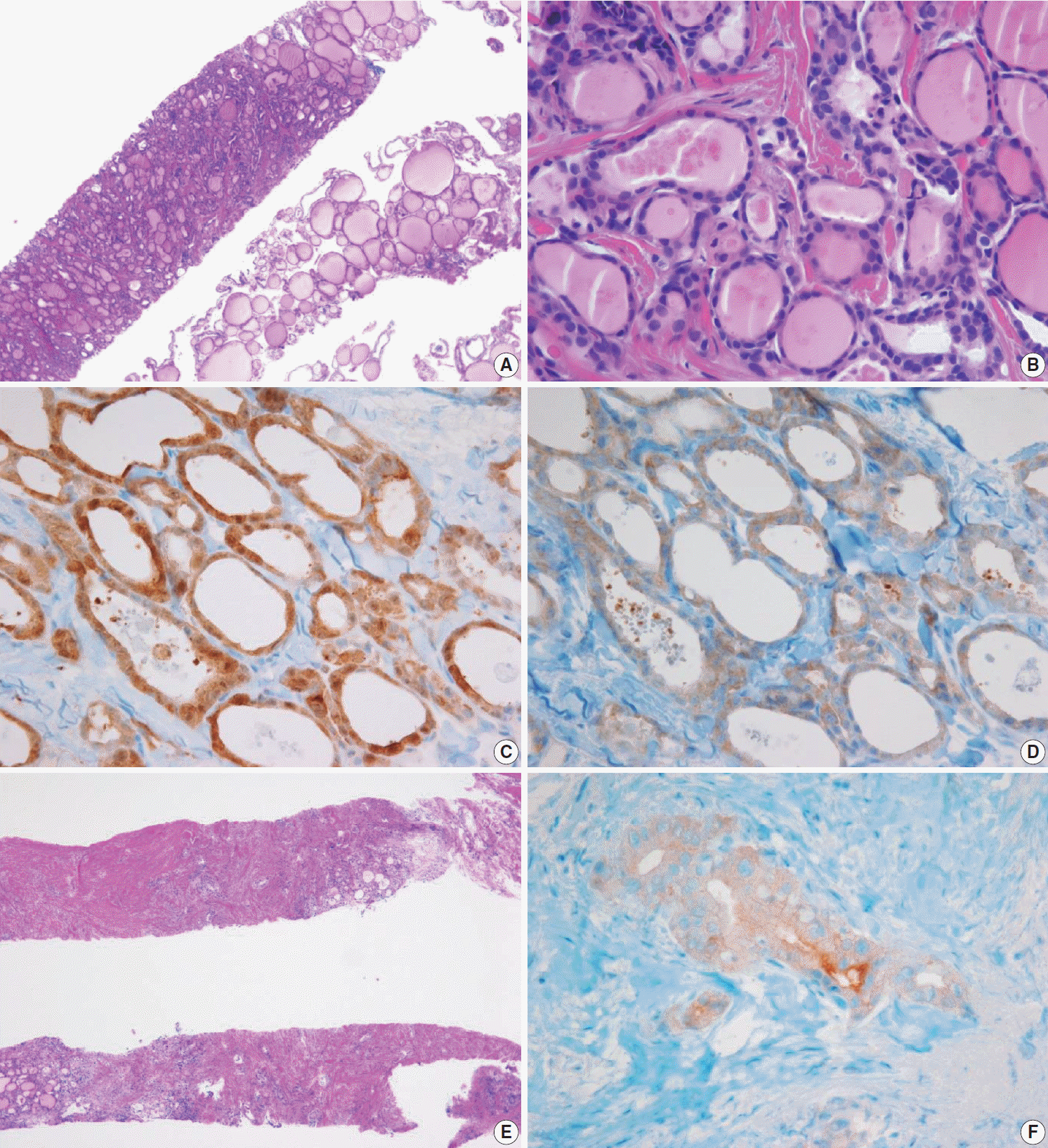
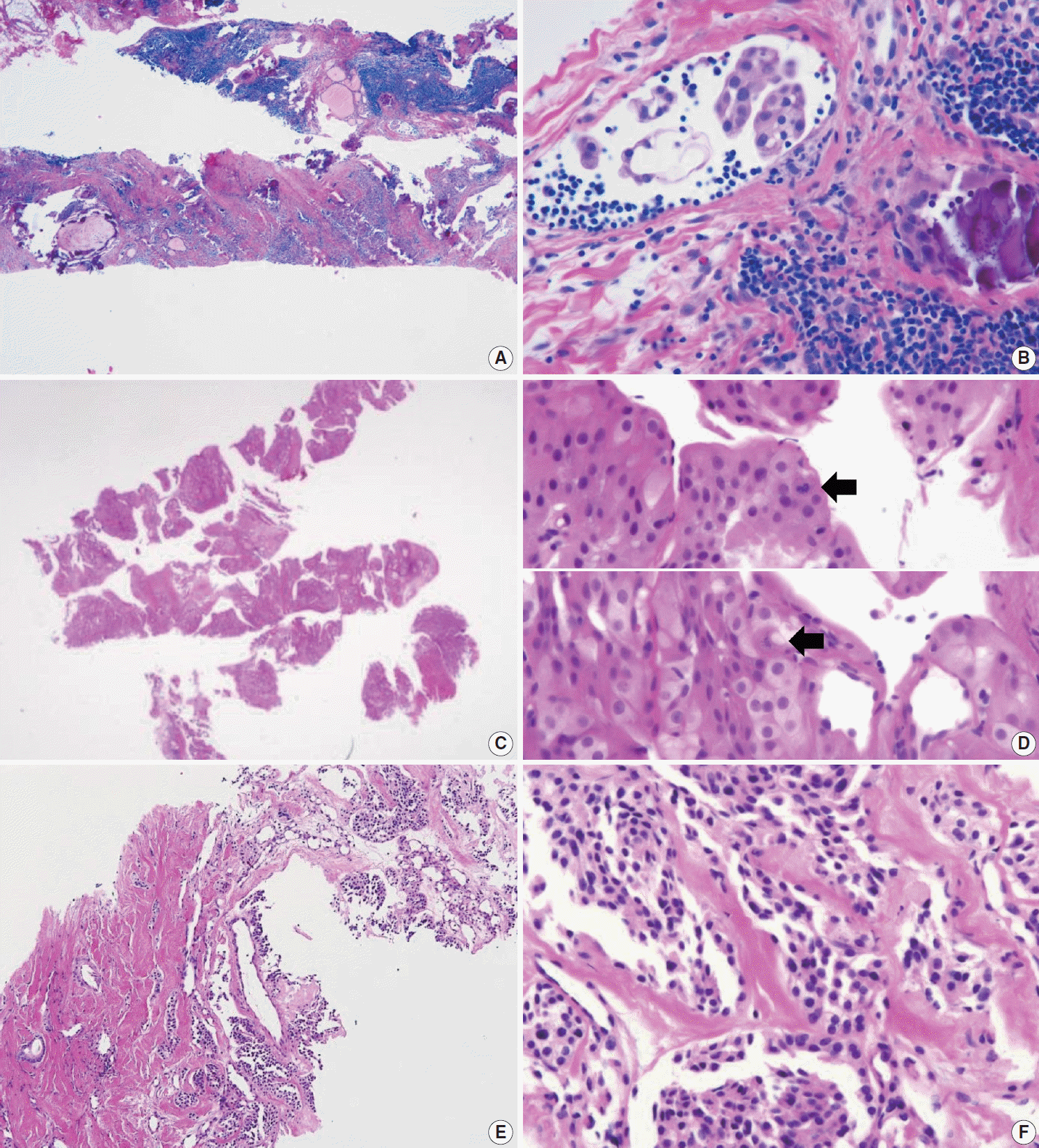

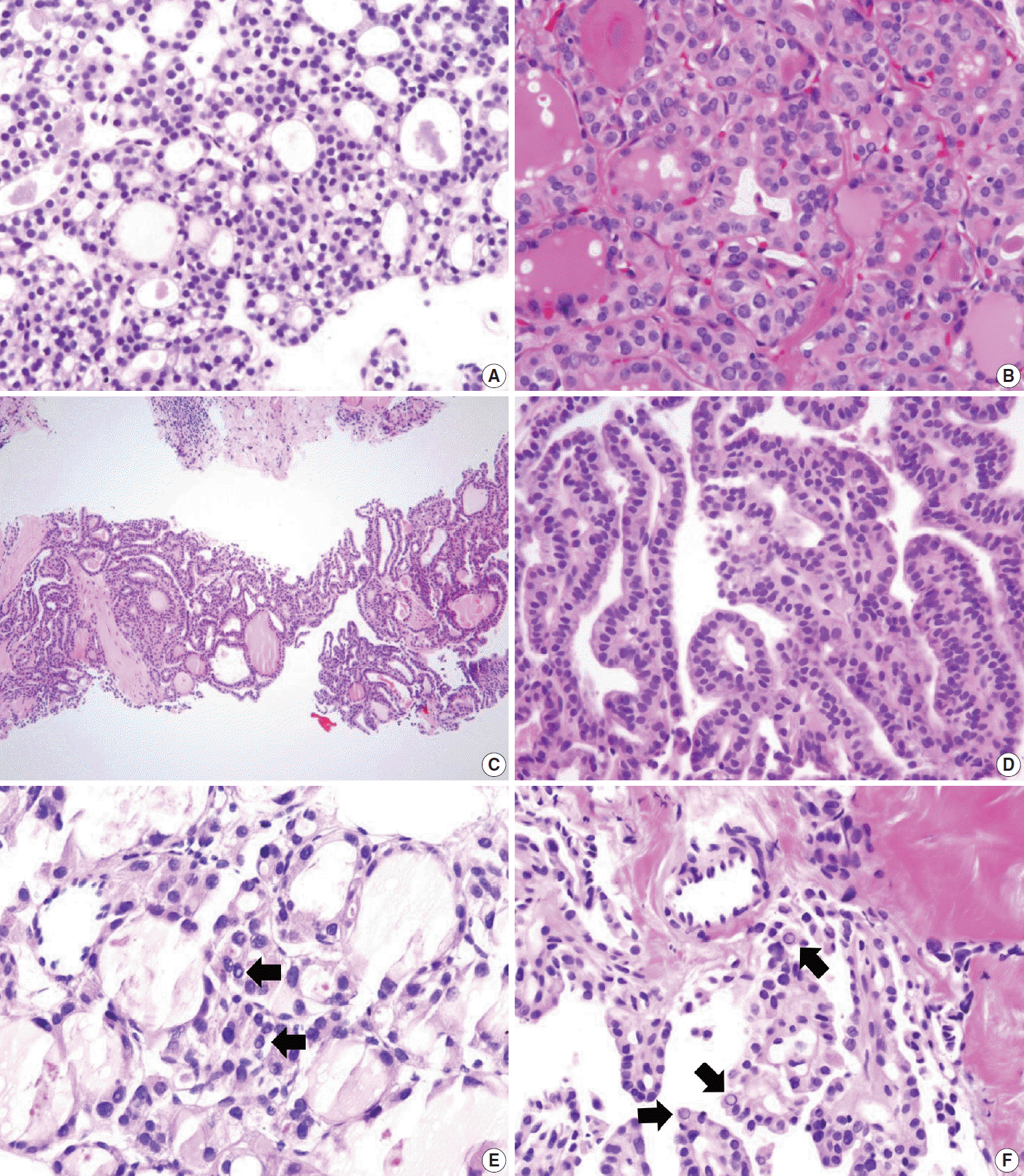




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download