Abstract
Background
The cytologic diagnosis of poorly differentiated thyroid carcinoma (PDTC) is difficult because it lacks salient cytologic findings and shares cytologic features with more commonly encountered neoplasms. Due to diverse cytologic findings and paucicellularity of PDTC, standardization of cytologic diagnostic criteria is limited. The purpose of this study is to investigate and recognize diverse thyroid findings of fine needle aspiration (FNA) cytology and frozen smear cytology in diagnosis of this rare but aggressive carcinoma.
Methods
The present study included six cases of FNA cytology and frozen smears of histologically diagnosed PDTCs.
Results
PDTC showed cytologic overlap with well-differentiated thyroid carcinomas (WDTCs). Five of six cases showed dedifferentiation arising from well differentiated thyroid carcinomas. Only one de novo PDTC showed highly cellular smears composed of discohesive small cells, high nuclear/cytoplasmic (N/C) ratio, prominent micronucleoli, and irregular nuclei. Retrospectively reviewed, these findings are highly suspicious for PDTC. Cytologic findings of nuclear atypia, pleomorphism, and irregularity were frequently found, whereas scattered small cells were seen only in the de novo case.
Conclusions
Heterogeneous cytologic findings of PDTCs are shared with those of WDTCs and contribute to difficult preoperative cytologic diagnoses. Most PDTCs show dedifferentiation from WDTCs. Albeit rare, de novo PDTC should be considered with cytology showing discohesive small cells with high N/C ratio. This will enable precise diagnosis and prompt treatment of this aggressive malignancy
Poorly differentiated thyroid carcinoma (PDTC) was first described by Sakamoto et al. [1] as an aggressive thyroid malignancy with morphology and biological behavior between those of well differentiated follicular or papillary thyroid carcinoma and undifferentiated anaplastic thyroid carcinoma (ATC). Recent genetic alterations may be helpful for predicting the biologic behavior of PDTC. Mutations in the promoter region of telomerase reverse transcriptase are detected in up to 40% of PDTCs, which is intermediate between mutation rates reported for well differentiated thyroid carcinoma (WDTC) and undifferentiated ATC [2]. Because PDTC requires additional chemoradiotherapy after surgical resection to prevent recurrence and metastasis, exact diagnosis is important [3].
PDTC is diagnosed using histologic criteria [4,5]. Unfortunately, most cases are diagnosed after surgical resection and not by preoperative fine needle aspiration (FNA) cytology [6]. PDTC is usually diagnosed from thyroidectomy specimens. According to the recent World Health Organization (WHO) classification, PDTC can only be definitively diagnosed histopathologically, but we believe that the Turin criteria applied to FNA specimens can aid in differentiation of PDTC from anaplastic carcinoma in clinically advanced cases. This was also described by Bongiovanni et al. [7]. The use of thyroid core needle biopsy is increasing, but FNA cytology remains the most commonly used first-line diagnostic tool for evaluating thyroid nodules [8]. Prompt preoperative diagnosis is important for planning multimodality treatment because PDTC has a greater propensity for recurrence or distant metastasis than differentiated thyroid carcinoma. However, presumptive cytologic diagnosis via FNA is challenging due to the rarity of PDTC and its overlapping features with various follicular neoplasms of the thyroid. Here, we describe and review cytologic findings of PDTCs.
Six cases of PDTC treated between from January 2015 to November 2018 at Gachon University Gil Medical Center, Incheon, South Korea were retrospectively analyzed. The thyroid FNA smears of all six cases were histologically diagnosed as PDTCs according to histologic criteria agreed upon at the Turin consensus conference held in 2006 [5]. Hematoxylin and eosin stained cytologic smears and liquid based cytology (ThinPrep Pap test, Cytyc Corporation, Boxborough, MA, USA) were evaluated. Diagnostic cytologic categorization of FNA was conducted using the six-level diagnostic scheme described in the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) [7]. Core needle biopsy performed in one case was classified based on the reporting procedure proposed by the Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group (2015) [9]. TNM staging was evaluated by the American Joint Committee on Cancer [10].
Case 1 was a 55-year-old man transferred from our neurology center due to an abnormal thyroid ultrasound during regular follow up. FNA was performed on a 3.3 cm oval hypoechoic mass. Six months later, the mass had increased to 4.4 cm. Core needle biopsy gave the impression of a follicular neoplasm, and total thyroidectomy was performed after frozen cytology and frozen section examination. The patient received radioactive iodine treatment and was free of recurrence at 7 months after surgery. Case 2 was a 35-year-old woman with an incidentally identified thyroid mass. On thyroid ultrasound, the mass was 4.9 cm sized, ill-defined, oval, hypoechoic, and located in the right lobe. FNA cytology was performed. Frozen cytology and frozen section findings were highly suspicious for insular carcinoma, and total thyroidectomy was performed. The case was diagnosed as PDTC with no nodal metastasis. High dose radioactive iodine treatment was planned. Three months after surgery, the patient was free of recurrence. Case 3 was a 65-year-old man diagnosed with papillary thyroid carcinoma at an outside hospital. Enhanced head and neck computed tomography showed a large mass measuring 6.8 cm at the right lobe and 4.7 cm at the isthmus. After FNA slides prepared at the outside hospital were reviewed, right lobectomy was performed. The patient refused further treatment, including total thyroidectomy, and was lost to follow up. Case 4 was a 39-year-old man who presented with an incidentally identified thyroid mass. Thyroid ultrasound showed a 1.8-cm-sized indeterminate mass in the right lobe. FNA followed by total thyroidectomy and radioactive iodine (150 mCi) treatment were performed. During 11 months of follow up, the patient was free of recurrence. Case 5 was a 73-year-old woman who presented with an incidental thyroid mass. Thyroid ultrasound showed a 5.5 cm predominantly solid heterogeneous mass in the left lobe. After FNA and total thyroidectomy, 131I radioactive iodine (150 mCi) was administered. During 45 months of follow up, she remained free of recurrence and continues to be followed every 6 months. Case 6 was a 66-year-old woman who presented with a long-standing left thyroid mass that replaced the mid and lower poles of the left thyroid. Preoperative FNA and intraoperative frozen cytology with frozen sections were performed and followed by total thyroidectomy. During 22 months of follow up, there was no evidence of tumor recurrence or lymph node metastasis.
FNA of case 1 showed a small amount of follicular cells with no mitosis or necrosis. The initial diagnosis was categorized as atypia of undetermined significance requiring the differential diagnoses of follicular neoplasm and follicular pattern-predominant papillary carcinoma (Fig. 1A). FNA cytology in case 2 revealed scattered, highly cellular, small round cells (Fig. 1C, D) and no evidence of nuclear inclusions, grooves, or necrosis. Initially, a follicular neoplasm was suspected. Because of the small scattered cells, a nodular form of lymphoid malignancy was also suspected. Frozen cytology and frozen sections raised a high level of suspicion for PDTC (Fig. 1E). FNA of case 3 revealed cellular clusters of atypical follicular cells with irregular vesicular nuclei; initial diagnosis was suspicious for papillary carcinoma, i.e. category V (Fig. 1F). FNA of case 4 showed a few scattered clusters of follicular cells with mild irregular nuclei in a hemorrhagic background (Fig. 1G) and was classified as category IIII, follicular lesion of undetermined significance. FNA of case 5 revealed paucicellular bloody smears (Fig. IH) suggestive of a benign follicular nodule. On retrospective review, aspirated smears were scant. Intraoperative frozen imprint smears revealed a few papillary structures in many singly scattered small cells showing occasional nuclear inclusions (Fig. 1I, J). FNA of case 6 showed scant cellularity and clusters of follicular cells with occasional oncocytic features and slight cellular atypia (Fig. 1K). This case was originally diagnosed as follicular neoplasm, Hurthle cell (oncocytic) type (diagnostic category IV). Frozen touch smears revealed scattered atypical small cells (Fig. 1L).
Histologically, core needle biopsy of case 1 showed a microfollicular proliferative lesion with a fibrous capsule and no mitosis or necrosis. The lesion was categorized as category IV, follicular neoplasm/suspicious for follicular neoplasm. Total thyroidectomy revealed a 4.4 cm oval mass with a PDTC portion (30%) arising from follicular carcinoma (Fig. 2A). The PDTC area showed frequent mitosis (9 per 10 high-power field [HPF]) and focal necrosis (Fig. 2B). Case 2 showed a large mass with scattered adjacent satellite nodules and extensive vascular invasion in the right lobe (Fig. 2C). A diffuse solid growth pattern with frequent mitotic activity and necrosis were observed (Fig. 2D). Histology of case 3 showed PDTC (10%) arising from follicular carcinoma and measuring 6.5 cm (Fig. 2E). Total thyroidectomy in case 4 revealed a PDTC portion (20%) with a solid and trabecular pattern that arose from a 1.3-cm well differentiated follicular carcinoma confined within the thyroid (Fig. 2F). In case 5, total thyroidectomy revealed PDTC arising from a follicular carcinoma (Fig. 2G) with necrosis (Fig. 2H). In case 6, total thyroidectomy revealed a large bulging ovoid mass replacing the left lobe. The mass was a 2.2-cm poorly differentiated carcinoma with a trabecular pattern that arose from follicular carcinoma (Fig. 2I). Due to complete capsule formation with capsular invasion, follicular neoplasm including widely invasive follicular carcinoma was suspected. Up to 11 mitoses per 50 HPF was observed in the PDTC area (Fig. 2J). Size ranged from 1.8 to 7.0 cm (median, 5.2 cm). Three females and three men were included, and ages ranged from 35 to 73 years (median, 56 years). Resected tumors ranged from 1.8 to 7.0 cm (median, 5.2 cm).
Clinical, cytologic, and histologic summary of the six cases is provided in Table 1.
PDTC has morphology and biological behavior between those of WDTC and ATC. PDTC either develops by dedifferentiation of WDTC or de novo from benign thyroid follicular cells. All of the present six cases except for case 2 showed PDTC dedifferentiated from WDTC. The current histologic criteria of PDTC are as follows [5]: (1) presence of solid, trabecular, or insular growth pattern, (2) absence of conventional nuclear features of papillary carcinoma, and (3) presence of at least one of the following features: convoluted nuclei, mitotic activity >3 per 10 HPF or 3 per 10 HPF, and tumor necrosis. These criteria achieved consensus in Turin in 2007 [4], and the recent WHO classification is also based on this consensus [7]. In the 2nd edition of the TBSRTC, cytologic criteria include a uniform population of malignant follicular cells with scant cytoplasm [7]. However, cytologic diagnostic criteria have not been standardized. The most recent edition of TBSRTC proposed the following criteria: (1) uniform population of malignant follicular cells with scant cytoplasm with/without oncocytic features, (2) high nuclear/ cytoplasmic (N/C) ratio with variable nuclear atypia, (3) presence of necrosis, apoptosis, or mitosis, and (4) scant colloid and cytoarchitecture of an insular, solid, or trabecular pattern. However, these FNA findings have no great specificity in explanatory notes. Previous studies on the FNA findings of PDTC showed no universally accepted cytologic criteria, and the issue remains problematic [7]. The presence of tumor necrosis or mitotic figures on FNA provided helpful diagnostic clues in previous studies [11]. However, these high grade cytologic findings are shared with those of ATC. Combined WDTC with PDTC and ATC are also difficult to diagnose by cytology [12]. Even rare cases of follicular adenoma without capsular/vascular invasion may exhibit small foci of PDTC transformation or spindle cells mimicking PDTC and are not easily extracted by FNA [1,13]. Cytologic findings of PDTC tend to overlap those of WDTC or even medullary thyroid carcinoma (MTC). Bongiovanni et al. [7] suggested that PDTC exhibits four major characteristic cytologic features: insular/solid/trabecular pattern, high N/C ratio, severe crowding, and single cells [6]. In addition, bare nuclei, polymorphonuclear leukocytes, and endothelial wrapping have also been suggested as ancillary diagnostic features [14]. In previous reviews of diverse cytologic studies, the most commonly reported FNA findings of PDTC were granular or salt-and pepper-like chromatin pattern, small cells with high N/C ratio, nuclear overlapping, and mild nuclear pleomorphism [15]. Thus, primitive, small tumor cells with scant cytoplasm and high N/C ratio are a key diagnostic feature of PDTC. The most important differential diagnoses of scattered small cells in PDTCs are papillary carcinoma and a small cell variant of MTC [14-16]. The classic morphology of MTC presents as epithelioid cells with occasional plasmacytoid appearance and focal spindle cell morphology. Giant, clear, and oncocytic cells, and small cell variants may also be observed. The small cell variant of MTC mimic resembles PDTC, Ewing sarcoma, lymphoma, metastatic carcinoma, or primary small cell carcinoma [17]. The small cell variant of MTC also exhibits granular plasmacytoid cytoplasm and a salt-andpepper-like nuclear chromatin pattern with occasional background amyloid; granular/coarse chromatin was reported in up to 95.5% of cases by Kane and Sharma [11] The oncocytic variant of PDTC should be distinguished from MTC, oncocytic (Hurthle cell) carcinoma, and metastatic carcinoma [15]. This rare variant of PDTC shows frequent small cell changes (as in case 2) and lymphoma, small cell carcinoma, and primary or metastatic neuroendocrine tumor should be included in differential diagnoses [17]. Necrosis or increased mitosis are rarely found in papillary carcinoma or well differentiated follicular carcinomas. These high grade cytologic features are more commonly found with undifferentiated thyroid carcinoma, but cytologic findings such as hypercellularity, insular pattern, small cell size, high N/C ratio, granular chromatin, severe nuclear overlapping, mild nuclear pleomorphism, abrupt nucleomegaly, apoptosis, mitosis, and necrosis are not observed in WDTC. Therefore, these diverse cytomorphologic features are crucial for cytologic diagnosis of PDTC.
Examination of smear backgrounds is also important for cytologic diagnosis of PDTC; hemorrhage without colloid and the occasional presence of necrotic debris may be important diagnostic clues. However, a background of necrotic debris is uncommon. In our six cases, only hemorrhagic background was found. Unfortunately, none of our FNA cytology cases fulfilled exact cytologic diagnostic criteria due to paucicellular, nonrepresentative samples (cases 1, 4, 5, and 6) or misinterpretation (cases 2 and 3). Case 3 was initially misinterpreted as papillary carcinoma; retrospective review showed small prominent micronucleoli and irregular nuclei that were overestimated as papillary carcinoma. TBSRTC suggests that the presence of isolated atypical follicular cells and focal necrosis and mitosis should be reported as suspicious for follicular neoplasm. A retrospective review of case 2 showed scattered discohesive small cells. However, the case should be considered highly suspicious for PDTC because dispersed scattered single cells or clusters of cells with a predominance of small cells is more commonly found in PDTC, MTC, and rarely solid variant of papillary carcinoma. The present six cases showed heterogeneous cytologic findings. Only one of six cases showed de novo PDTC without the WDTC component; small foci of PDTC can interfere with precise cytologic diagnosis. WDTC components comprise most of the tumors and interfere with precise preoperative cytologic examination. As shown in Table 1, one case was originally diagnosed as category II (case 5), one was diagnosed as category V (case 3), two were diagnosed as category IV (cases 2 and 6), and the remaining two were diagnosed as category III (cases 1 and 4). This variable cytologic categorization is in line with a review by Saglietti et al. [6]. Initial cytologic diagnosis in cases 2 and 6 was category IV, i.e., follicular neoplasm/suspicious for follicular neoplasm, which is plausible because PDTC shares monotonous uniform tumor cells with well differentiated follicular adenoma/carcinoma. In case 5, cytologic misdiagnosis as category II was caused by inadequate sampling of scarce cellularity. According to TBSRTC, 1/3 of PDTCs are reported as follicular neoplasm/suspicious for follicular neoplasm (category IV), and only 1/3 cases are reported as PDTC, or poorly differentiated carcinoma, not otherwise specified [18].
TBSRTC is now applied in preoperative FNA diagnosis and has improved the quality of reporting by decreasing diagnostic discrepancies and facilitating consistency in management plans. However, as shown above and in previous cytologic studies, the lack of consensus and standardization in FNA cytology of PDTC leads to ambiguity in both cytologic diagnoses and clinician interpretation of these diagnoses. More accurate and improved reporting of PDTC is anticipated with a recently suggested modified Bethesda system informing cytologic adequacy [18,19]. Previous studies correlating clinical information, cytomorphology, and ancillary immunohistochemical stains such as Pax8, BRAF, or thyroglobulin report improved diagnostic accuracy in thyroid FNA cytology, but these studies are theoretical and inconsistent [20].
In TBSRTC, inadequate samples caused by paucicellularity, cystic fluid only, obscuring blood, drying artifact, or calcified material result in low sensitivity and high false-negative rates. Adequacy criteria may be important to establish a benign category and minimize false negative results. Paucicellularity is caused by a small component of PDTC in most cases, and standardization of cytologic diagnostic criteria is limited. However, nuclear atypia, pleomorphism, and irregularity are frequent cytologic findings of PDTC. The mitosis and necrosis that are frequently found on histology are uncommonly encountered on FNA because most cases have small foci of PDTC. Only one of the six present cases had scattered poorly differentiated small cells, a helpful cytologic finding. Preoperative core needle biopsy may be non-representative because of a relatively small proportion of PDTC, leading to misdiagnosis. Cytologic diagnostic accuracy for PDTC is challenging and should be improved by recognizing its diverse cytologic findings.
Notes
REFERENCES
1. Sakamoto A, Kasai N, Sugano H. Poorly differentiated carcinoma of the thyroid: a clinicopathologic entity for a high-risk group of papillary and follicular carcinomas. Cancer. 1983; 52:1849–55.

2. Ibrahimpasic T, Ghossein R, Shah JP, Ganly I. Poorly differentiated carcinoma of the thyroid gland: current status and future prospects. Thyroid. 2019; 29:311–21.

3. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; 26:1–133.
4. Volante M, Collini P, Nikiforov YE, et al. Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol. 2007; 31:1256–64.

5. Tallini G, Asioli S, Aubert S, et al. Poorly differentiated thyroid carcinoma. In : Lloyd RV, Osamura RY, Kloppel G, Rosai J, editors. WHO classification of tumours of endocrine organs. Lyon: IARC Press;2017. p. 100–3.
6. Saglietti C, Onenerk AM, Faquin WC, Sykiotis GP, Ziadi S, Bongiovanni M. FNA diagnosis of poorly differentiated thyroid carcinoma: a review of the recent literature. Cytopathology. 2017; 28:467–74.

7. Bongiovanni M, Fadda G, Faquin WC. Poorly differentiated thyroid carcinoma. In : Ali SZ, Cibas ES, editors. The Bethesda System for Reporting Thyroid Cytopathology: definitions, criteria, and explanatory notes. 2nd ed. Cham: Springer International Publishing;2018. p. 177–88. 2nd.
8. Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: Executive Summary of recommendations. J Endocrinol Invest. 2010; 33:287–91.

9. Jung CK, Min HS, Park HJ, et al. Pathology reporting of thyroid core needle biopsy: a proposal of the Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group. J Pathol Transl Med. 2015; 49:288–99.

10. Amin MB, Edge S, Greene F, et al. AJCC cancer staging manual. 8th ed. Chicago: Springer;2017.
11. Kane SV, Sharma TP. Cytologic diagnostic approach to poorly differentiated thyroid carcinoma: a single-institution study. Cancer Cytopathol. 2015; 123:82–91.

12. Bichoo RA, Mishra A, Kumari N, et al. Poorly differentiated thyroid carcinoma and poorly differentiated area in differentiated thyroid carcinoma: is there any difference? Langenbecks Arch Surg. 2019; 404:45–53.

13. Burke A, Staats P. Follicular adenoma of the thyroid with spindle cell metaplasia mimicking insular thyroid carcinoma and lacking thyroglobulin expression. Am J Surg Pathol. 2019; 24:84–6.
14. Onenerk M, Canberk S, Gunes P, Erkan M, Kilicoglu GZ. Oncocytic variant of poorly differentiated thyroid carcinoma: “Is diagnosis possible by fine-needle aspiration?”. Cytojournal. 2016; 13:23.

15. Laforga JB, Cortés VA. Oncocytic poorly differentiated (insular) thyroid carcinoma mimicking metastatic adenocarcinoma: a case report and review of the literature. Diagn Cytopathol. 2019; 47:584–8.

16. Verma A, Kane S, Vinarkar S, D'Cruz AK. Small cell medullary thyroid carcinoma: a diagnostic dilemma. Indian J Pathol Microbiol. 2017; 60:562–4.

17. Eloy C, Cameselle-Teijeiro JM, Rousseau E, Sobrinho-Simões M. Small cell tumors of the thyroid gland: a review. Int J Surg Pathol. 2014; 22:197–201.
18. Lee YB, Kim JY, Cho H, et al. Modified Bethesda system informing cytopathologic adequacy improves malignancy risk stratification in nodules considered benign or atypia(follicular lesion) of undetermined significance. Sci Rep. 2018; 8:13503.

Fig. 1.
Cytologic findings of poorly differentiated thyroid carcinoma. (A) Case 1. Fine needle aspiration (FNA) shows few clusters of follicular cells with atypia. (B–E) Case 2. (B) FNA shows few scattered sheets or singly scattered small cells with no nuclear inclusions or grooves. (C–E) Frozen imprint cytology of intraoperative excised mass shows round tumor cells containing scant cytoplasm and round heterochromatic nuclei (C, D). (E) High power view shows atypical follicular cells with small prominent nucleoli. (F) Case 3. FNA reveals cellular clusters of atypical follicular cells with irregular vesicular nuclei. (G) Case 4. FNA of case 4 shows few scattered clusters of follicular cells with mildly irregular nuclei. (H) Case 5. FNA of case 5 shows scant cellular smear with few microfollicles. (I, J) Frozen imprint smear of case 5 shows few papillary structures (I) with nuclear atypia and occasional nuclear inclusions (J). (K, L) Case 6. (K) FNA shows cluster of atypical follicular cells with prominent nucleoli. (L) Intraoperative frozen smears show scattered small cells with nuclear irregularities.
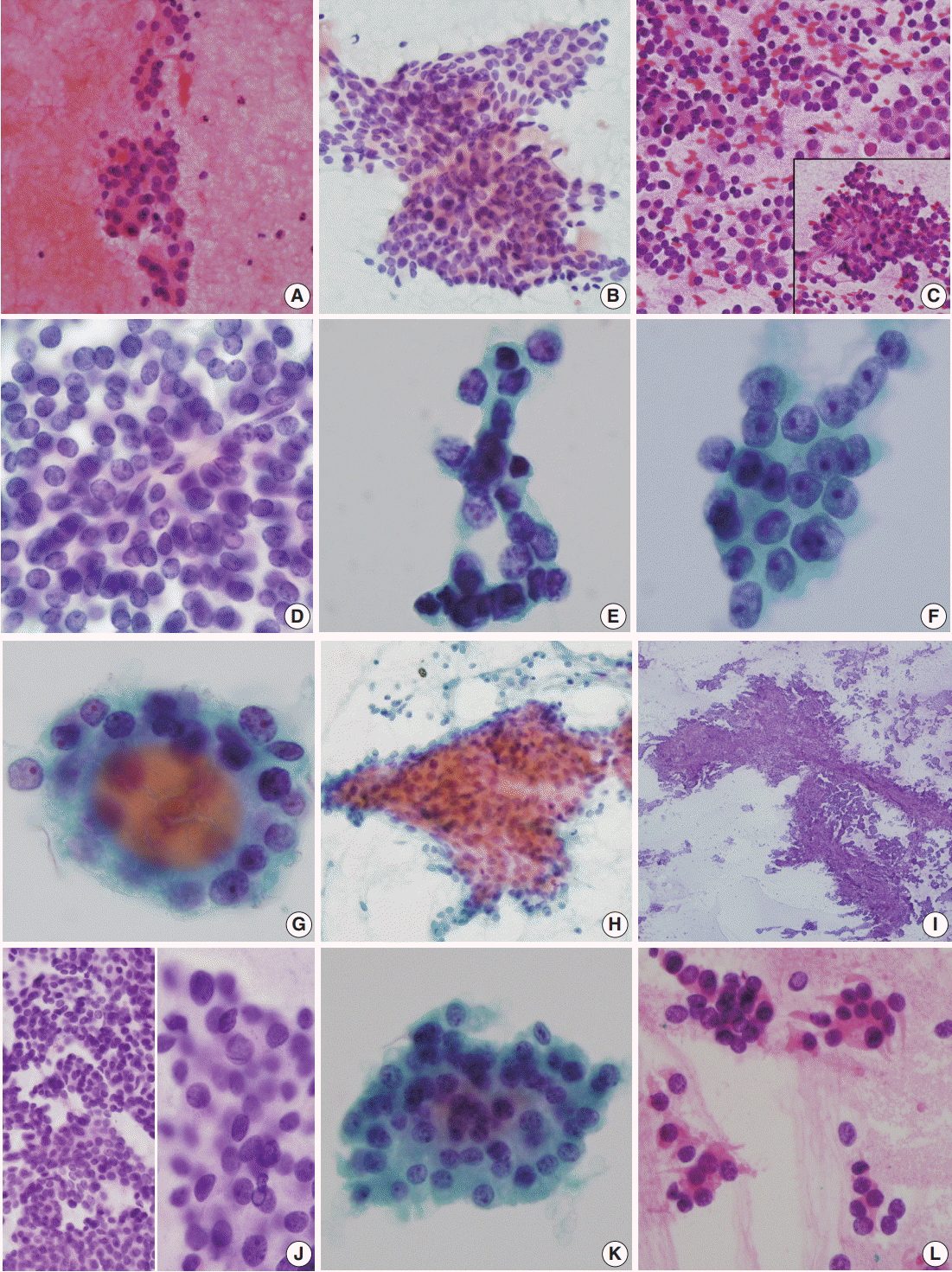
Fig. 2.
Histology of resected poorly differentiated thyroid carcinomas. (A, B) Case 1. Mass with well-demarcated capsule filled with microfollicles with necrosis. (B) Necrosis. (C, D) Case 2. Histology shows large mass with solid growth pattern and multifocal necrosis in insular pattern (C). Frequent mitotic activity (D, left) and coagulation necrosis (D, right) are observed. (E) Case 3. Histology shows follicular carcinoma and intervening necrosis with focal poorly differentiated carcinoma (inset). (F) Case 4. Histology shows extensive necrosis in well-differentiated follicular carcinoma. Inset shows cellular atypia adjacent to necrosis. (G, H) Case 5. (G) Solid growth pattern in follicular carcinoma. (H) High power view shows necrosis with transition to poorly differentiated carcinoma. (I, J) Case 6. (I) Histology of total thyroidectomy shows background well-differentiated follicular carcinoma and foci of poorly differentiated carcinoma. (J) High power view (left) is shown. Note mitosis in tumor cells (right).
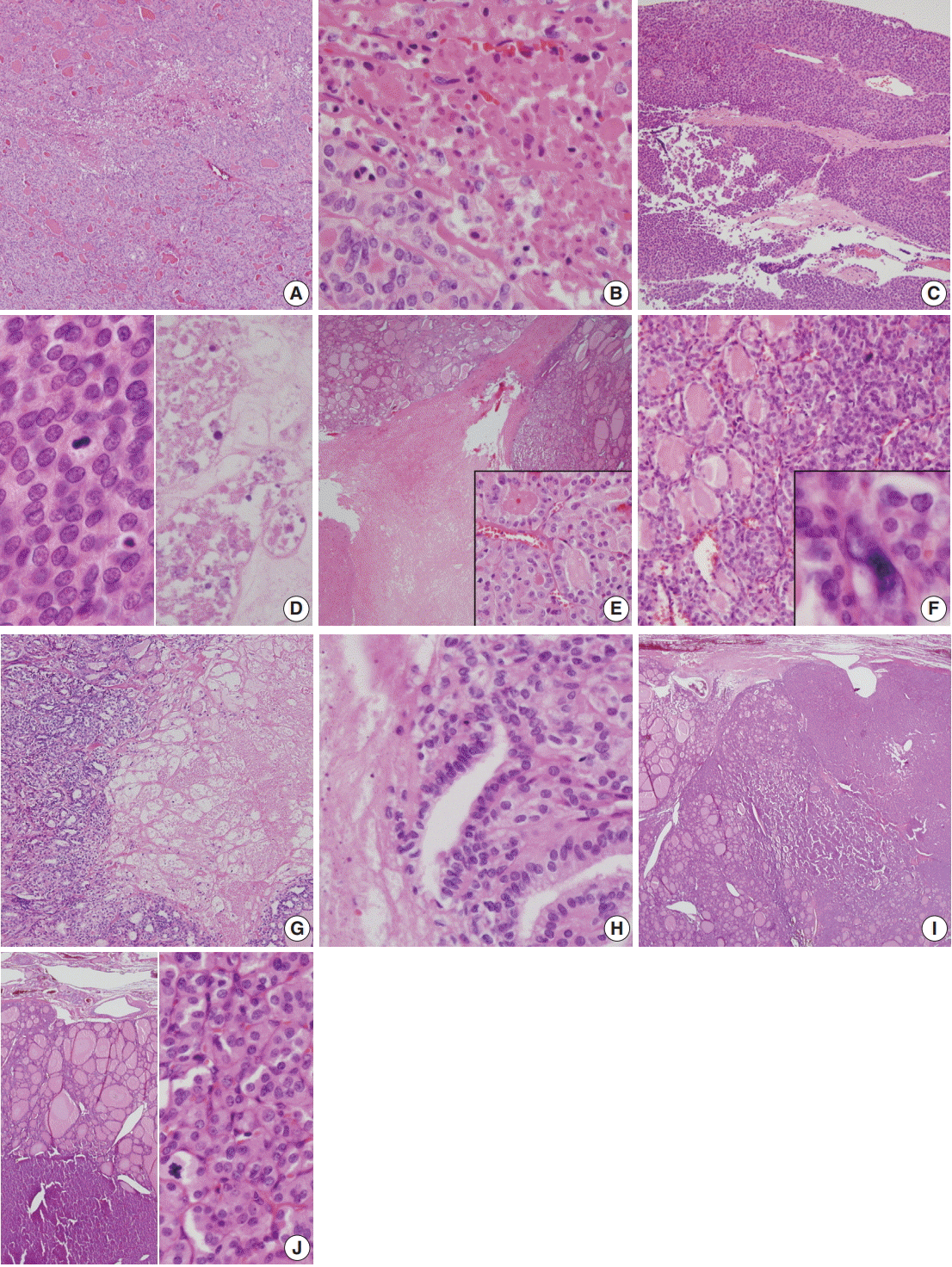
Table 1.
Clinicopathologic summary of six cases of poorly differentiated thyroid carcinoma




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download