Primary urothelial carcinoma (UC) of the prostate is rare, representing only about 1% to 4% of all prostate malignancies [
1-
3]. In contrast, secondary involvement of primary bladder UC is relatively common with an incidence of 12% in an early report [
4]. However, the true incidence of prostatic involvement is more common. In several studies using whole-mount prostate sectioning technique, the incidence of prostatic involvement has been reported up to 48% (average, 35.5%) [
5-
9]. The incidence of prostatic stromal invasion (PSI) ranges from 7.6% to 16.6% [
4,
6,
9-
13]. In 7th American Joint Committee on Cancer (AJCC) staging system, primary bladder UCs with PSI are classified as pT4a and correlated with worse prognosis [
14]. However, the staging of continuous subepithelial PSI from in situ carcinoma was not designated properly. The 8th AJCC staging system clarified the staging of PSI according to the mode of invasion: primary bladder UCs with direct PSI via transmural route is designated as T4a while continuous subepithelial PSI from in situ carcinoma is now classified as T2 [
15]. Although the presence of PSI does not affect the current 8th AJCC staging of muscle-invasive primary bladder UCs (≥T2), it is still important to distinguish PSI from in situ UC because PSI is associated with poor prognosis [
16]. Therefore, the correct identification of PSI with primary bladder UC is of paramount importance for accurate staging and predicting patients’ prognoses. However, the distinction between PSI by UCs from only in situ UC involvement of prostatic ducts or acini in the prostate can be challenging on hematoxylin and eosin (H&E) stained slides examination alone in some cases.
In this study, double cocktail immunostains with high molecular weight cytokeratin (HMWCK) and GATA-3 were performed to determine its usefulness for detecting PSI.
RESULTS
Clinicopathological data on these seven patients with equivocal PSI are summarized in
Table 1. The patients’ mean age was of 73.3 years at diagnosis (range, 60 to 93 years). Among seven cases, six showed conventional muscle invasive high-grade UC and one showed diffuse and multifocal UC in situ throughout the bladder (case 1). Six showed conventional high-grade UC morphology, but one case (case 4) showed several different histologic features (squamous, glandular and neuroendocrine differentiations). Lymphovascular invasion was identified in three cases. Five cases were free of tumor at margins and two showed positive margins, one in left ureter and the other in the urethra.
In these seven cases, UC involvement was seen in prostatic ducts and acini with equivocal PSI. Four cases revealed pattern 1 equivocal invasion (
Fig. 2A) and three cases pattern 2 equivocal invasion (
Figs. 2B,
3A,
B). Originally, two cases, both with pattern 2 equivocal invasion, were diagnosed as having PSI and the remaining five cases, four pattern 1 and one pattern 2 cases, with equivocal invasion were diagnosed as no PSI.
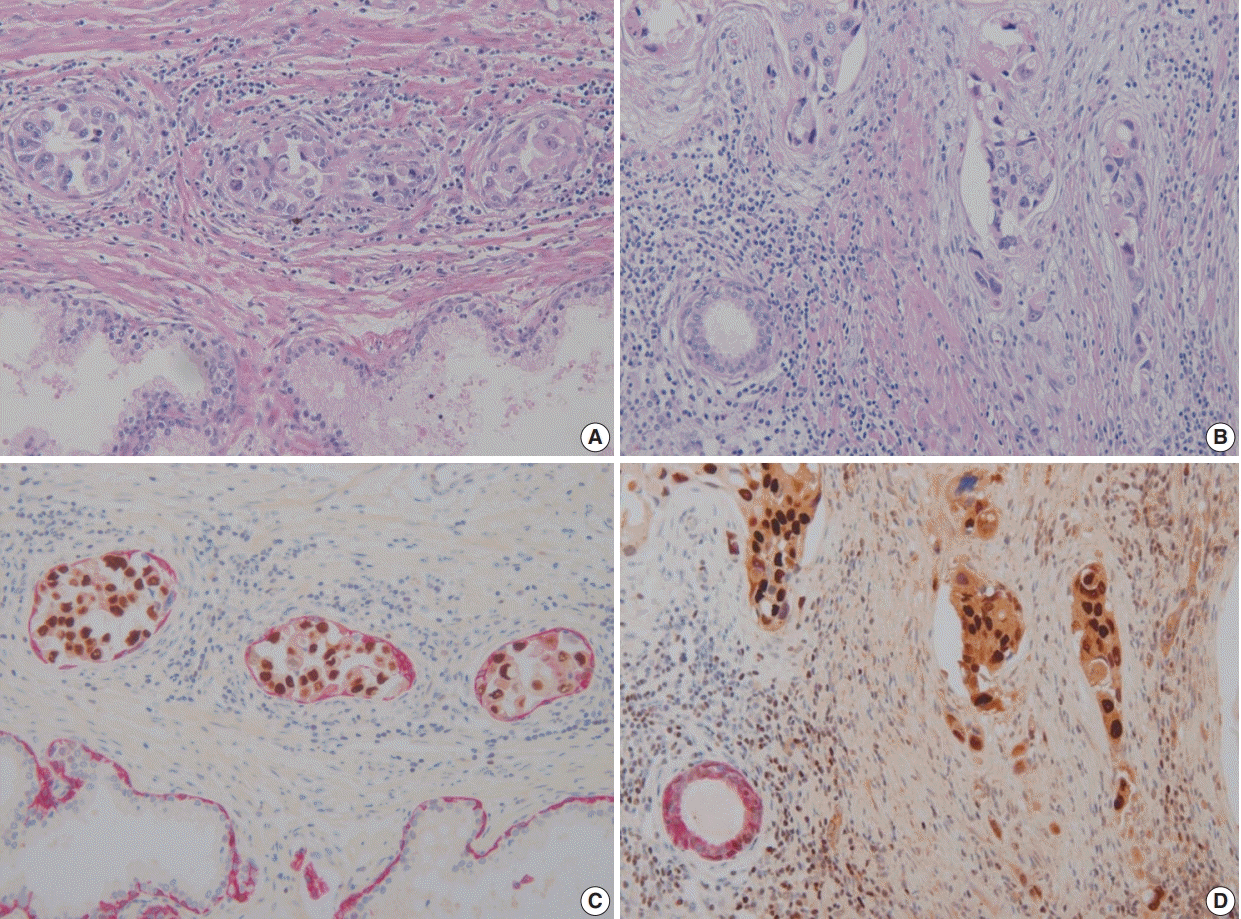
In these seven cases, double cocktail immunostains were performed. Prostatic basal cells surrounding in situ UC stained positively for HMWCK and UC cells stained for GATA-3 (
Fig. 1A,
C). In all cases, UC cells stained positively for GATA-3 (7/7, 100%). The combination of HMWCK (red)/GATA-3 (brown) easily distinguish in situ from invasive tumor (
Fig. 1B,
D). Among seven equivocal cases, two cases showed PSI and five cases in situ UC in the prostate. Based on these stain results, cases 5 and 6 were revised according to the 7th AJCC staging system and cases 5 and 7 were revised according to the 8th AJCC system. In one case, the original T4a classification was revised as T1 and in the other case, the original T3 classification was revised to T4a according to the 7th AJCC staging system. According to the 8th AJCC staging system, one case classified originally as T4a was revised as T1, and the other T4a case was revised as T2.
All patients received radical cystoprostatectomy after transurethral resection of bladder tumor and additional treatments such as bacillus Calmette-Guerin (BCG) instillation, neoadjuvant chemotherapy and adjuvant chemotherapy were given as needed. Three patients (cases 1, 7, and 5) are alive with disease at 21, 26, and 54 months, respectively. Other three patients (cases 4, 3, and 6) died of disease at 2-, 10-, and 16-month follow-up, respectively, and the remaining one patient (case 2) was lost to follow up.
In equivocal cases with pattern 1, GATA-3–positive tumor cells in prostatic ducts/acini were surrounded by HMWCK-positive basal cells (
Fig. 2C). In three equivocal cases with pattern 2, two cases were revised after performing double immunohistochemistry (IHC). In one case, double IHC revealed the presence of preserved basal cells with continuous, linear and focally weak staining pattern of HMWCK at the periphery of the involved prostatic ducts/acini (case 5) (
Fig. 2D). This case, originally signed out as PSI, was revised as in situ UC involving prostatic ducts/acini without PSI. In the other case (case 6), intense aggregates of inflammatory cells were identified adjacent to prostatic ducts (
Fig. 3A,
B). GATA-3–positive and HMWCK-negative singly scattered invasive tumor cells were present within the periductal inflammatory area (
Fig. 3C). Singly scattered tumor cells were also highlighted by pancytokeratin stain, supporting invasive UC cells (
Fig. 3D). This case, originally signed out as in situ UC involving prostatic ducts, was revised as having PSI.
DISCUSSION
It is well-known that UCs with extensive PSI has a poor prognosis, but even a few cases with focal PSI may have similarly poor prognosis [
16,
17]. Therefore, it is essential to find even focal areas of PSI for proper classification. In 7th AJCC staging system, primary bladder UCs invading prostatic stroma through spreading to the prostatic urethra are classified as T4a. However, in the new 8th AJCC staging system [
15], only those cases with primary bladder UCs directly invading prostatic stroma through the bladder wall are classified as T4a. Continuous subepithelial PSI from in situ UC is now categorized as T2 and does not affect the staging of primary bladder muscle-invasive UC (> T2). Yet distinguishing continuous subepithelial PSI of in situ UC (T2) from involvement of in situ UC (Tis) in non-muscle invasive primary bladder UCs (Tis or T1) are still important for patients who underwent early radical cystoprostatectomy because overall tumor stage can be upstaged because of the presence of PSI. Also, even though the impact of a concurrent continuous subepithelial PSI on the prognosis of muscle-invasive primary bladder UCs (> T2) is still unclear, the presence of PSI itself is reported to be associated with poor prognosis regardless of the mode of invasion [
16].
Therefore, the significance of PSI needs to be re-evaluated. In most cases with PSI, stromal invasion can be easily detected on H&E slides because PSI is usually associated with single isolate cell invasion, desmoplastic stromal reaction, acute and/or chronic inflammatory response, and retraction artifact around tumor cells [
18]. However, the determination of PSI is somewhat subjective and may be impossible in some cases because they lack these characteristic histologic features on H&E slides. Perrino et al. [
19] showed 11.1% (4/36) discordant interpretations of PSI between original report and review using double immunostains with cytokeratin (CK) 7/CK5 and p53/CK5 [
20]. The main distinction between in situ UC from PSI is to identify tumor cells in the prostatic stroma without surrounding prostatic basal cells.
In this study, we evaluated the utility of HMWCK and GATA-3 double IHC in determining stromal invasion of primary bladder UC in the prostate. We classified equivocal PSI cases into two patterns on H&E. In all cases with pattern 1 invasion, the diagnoses based on H&E and on IHC were concordant. However, in cases showing pattern 2, the concordance rate was low with two of three cases being misdiagnosed on H&E. Singly scattered tumor cells were present in inflammatory stroma in those two cases. The singly scattered tumor cells were positive for pancytokeratin as well as GATA-3 but negative for HMWCK. Based on these findings, we recommend pancytokeratin and GATA-3 IHC stains in the prostate with pattern 2 equivocal PSI.
In previous two studies, various combinations of double stains were used to differentiate in situ UC from PSI in the prostate [
18,
20]. Chastain et al. [
18] demonstrated that p63 and HMWCK double stains are useful in differentiating PSI. Even though this combination of double IHC is useful when p63-positive tumor cells are present in the prostatic stroma without surrounding HMWCK-positive basal cells, it has a limitation because not all tumor cells are positive for p63. In all cases, basal cells showed strong staining for both p63 and HMWCK, but only 50% and 41% of cases showed no or weak expression in tumor cells, respectively [
18]. In the other study, Fichtenbaum et al. [
20] reported that double stains with CK7/CK5 and p53/CK5 discriminated in situ vs. invasive urothelial cancer in the prostate. These combinations of double stains are also a good method to discriminate in situ vs. PSI and can be utilized in a routine practice because CK5 is a very sensitive and specific marker for basal cells (100%) and CK7 and p53 are very sensitive markers for tumor cells with 100% and 83% positivity, respectively [
20]. CK7 is very specific for UCs in differentiating commonly encountered adenocarcinomas such as prostatic adenocarcinoma and metastatic colonic adenocarcinoma because the expression of CK7 is not usually seen in these adenocarcinomas [
21,
22]. However, even though rare, other metastatic adenocarcinomas including pulmonary adenocarcinoma, ovarian serous adenocarcinoma, endometrial adenocarcinoma, gastric adenocarcinoma, cholangiocarcinoma, invasive ductal carcinoma, that can be found in the prostate also may express CK7 [
21,
22]. Moreover, high grade UC may be negative for CK7 occasionally [
23]. In our study, we used GATA-3 stain for UCs and HMWCK for prostatic basal cells. GATA-3 is a recently described immunohistochemical marker that is specific and sensitive for UCs and breast cancers [
24,
25]. When GATA-3–positive tumor cells are present in the prostatic stroma, PSI by UCs is highly suggested in the proper clinical context.
There are several limitations in this study. First, selective limited prostate sectioning technique was applied to this study. If whole-mount step sections of the prostate were selected, more cases with prostatic involvement might have been detected. Second, the number of subjects, especially the number of cases with equivocal PSI, was rather small. Third, GATA-3 also stains lymphocytes. GATA-3-positive atypical cells were present in the inflammatory stroma (case 6). Even though GATA-3–positive atypical cells in the inflammatory stroma were confirmed as epithelial cells by pancytokeratin staining, they might be keratin-positive debris such as disrupted normal epithelium or disrupted prostatic ducts or glands, especially in post-BCG status. However, BCG instillation was not performed in this case. Fourth, GATA-3 is recently reported to show aberrant diffuse nuclear staining in both luminal and basal cells of benign prostate glands with radiation atypia as well as in the basal cells of non-irradiated benign prostate glands [
26]. UC and benign prostate glands can be distinguished based on their morphology, but certain variants of UCs, especially UC with glandular differentiation can cause a diagnostic problem. Fifth, even though GATA-3 is a novel immunohistochemical marker for UC, GATA-3 expression differed among UC variants [
27]. Especially, GATA-3 is reported to be weakly or rarely expressed in sarcomatoid and small cell carcinoma variants of UC and squamous cell carcinomas [
27]. Thus, GATA-3 immunostain may have only limited diagnostic value in such cases. Lastly, strong HMWCK positivity is reported in 50% to 91.7% of UCs [
18,
28].
In the current study, weak GATA3 positivity in basal cells of benign prostatic glands was observed in two out of seven cases (28.6%) while HMWCK positivity in UCs was found in five out of seven (71.4%). Such expression pattern could cause diagnostic confusion for pathologists in irradiated specimens which often accompany radiation atypia in benign prostatic glands. However, none of the patients in the current study had received previous irradiation therapy. Also, even though some benign prostatic glands expressed GATA3 positivity, the staining intensity was much weaker than that observed in UCs. Furthermore, none of GATA3 positive benign prostatic cells in the current study showed cytologic atypia.
The double staining for GATA-3 and HMWCK may enable us to distinguish PSI from equivocal in situ lesions. In rare cases in which GATA-3 is aberrantly expressed in prostatic glands, the presence of HMWCK staining and the architectural and cytological findings on H&E can aid in the correct interpretation. Another advantage of the double staining method is convenience. Compared to switching between GATA-3 and HMWCK stains individually to spot the equivocal lesion under microscopic examination, assessing a single slide is more convenient. Lastly, the equivocal PSI lesions tend to be subtle and minute in size. Double immunostain method reduces the possible loss of the lesion during multiple sections for different markers.
Differentiating cases of UC with PSI from in situ UC involving prostatic ducts or acini is critical for subsequent patients’ prognosis even though the significance of focal PSI is still controversial and PSI itself is not considered as pT4a any longer in 8th AJCC staging system. However, one patient with PSI (case 6, original T3 [7th]→revised to T4a [7th], T3 [8th]) died with only 16 months follow-up after cystoprostatectomy. On the contrary, the other patient without PSI (case 5, original T4a [7th]→revised to T1 [7th], T1 [8th]) is still alive for 54 months. In these equivocal cases, one case (case 5) was misdiagnosed as PSI and subsequently T4a (7th AJCC) was assigned in the original diagnosis. The presence of basal cells around tumor nests, as demonstrated by double IHC, led to the final diagnosis of in situ UC involving prostatic ducts in this case. The other case (case 6) was diagnosed as in situ involving only prostatic ducts without PSI in the original diagnosis; therefore, pT3 was based on the perivesical fat invasion of the primary bladder UC. However, PSI besides in situ prostatic duct involvement was diagnosed because scattered infiltrating tumor cells with positive GATA3 and pancytokeratin positivity with lack of HMWCK stain within the inflammatory infiltrates and subsequently pT4a was re-assigned in this case. Another patient with PSI (case 7, original T4a [7th]→revised to T4a [7th], T2 [8th]) is also alive for 58 months. In this case, the primary bladder UC was non-muscle invasive and the concurrent urethral PSI determined the staging.
In conclusion, we demonstrated that GATA-3 and HMWCK double IHC is useful in discriminating PSI by UC from in situ UC involving prostatic ducts or acini especially in cases with pattern 2 equivocal PSI.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download