MATERIALS AND METHODS
Specimens and patient selection
Automated immunohistochemistry
Evaluation of immunohistochemistry
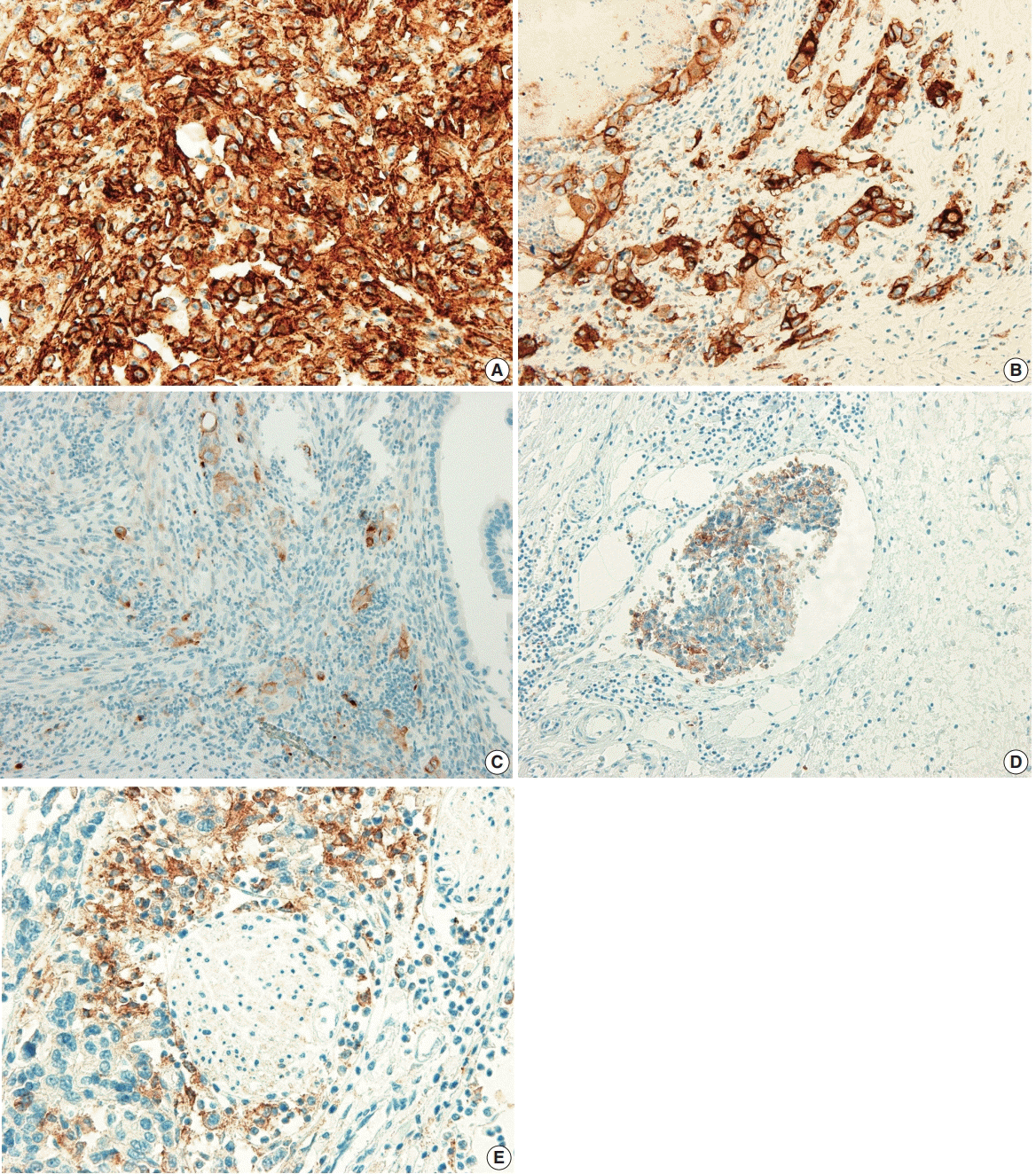 | Fig. 1.Programmed death-ligand 1 (PD-L1) expression in the tumor cells of gallbladder cancer (GBC). (A) ≥ 50% positive staining of PDL1 in tumor cells. (B) 10% to 49% positive staining of PD-L1 in tumor cells. (C) 1% to 9% staining of PD-L1 in tumor cells. (D) Positive staining of PD-L1 in tumor cells of lymphovascular invasion. (E) Positive staining of PD-L1 in tumor cells of perineural invasion. |
Statistical analysis
Ethics statement
RESULTS
Clinicopathological characteristics
Table 1.
Correlation of clinicopathological parameters with PD-L1 expression
Table 2.
| Clinicopathological parameter |
PD-L1 |
p-value |
PD-L1 |
p-value |
PD-L1 |
p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| < 1% | ≥ 1% | < 10% | ≥ 10% | < 50% | ≥ 50% | ||||
| Sex | .887 | .270 | > .99a | ||||||
| Male | 36 (43.9) | 8 (42.1) | 36 (41.4) | 8 (57.1) | 41 (44.1) | 3 (37.5) | |||
| Female | 46 (56.1) | 11 (57.9) | 51 (58.6) | 6 (42.9) | 52 (55.9) | 5 (62.5) | |||
| Age (yr) | .259 | .302 | .716a | ||||||
| < 68 | 42 (51.2) | 7 (36.8) | 44 (50.6) | 5 (35.7) | 46 (49.5) | 3 (37.5) | |||
| ≥ 68 | 40 (48.8) | 12 (63.2) | 43 (49.4) | 9 (64.3) | 47 (50.5) | 5 (62.5) | |||
| Histologic type | .132a | .004a | .306a | ||||||
| Adenocarcinoma NOS, ICPN with associated invasive carcinoma | 73 (89.0) | 14 (73.7) | 79 (90.8) | 8 (57.1) | 81 (87.1) | 6 (75.0) | |||
| Adenocarcinoma with other component, others | 9 (11.0) | 5 (26.3) | 8 (9.2) | 6 (42.9) | 12 (12.9) | 2 (25.0) | |||
| Histologic grade | .001 | < .001a | < .001a | ||||||
| Well differentiated | 42 (51.2) | 3 (15.8) | 44 (50.6) | 1 (7.1) | 45 (48.4) | 0 | |||
| Moderately differentiated | 28 (34.1) | 6 (31.6) | 31 (35.6) | 3 (21.4) | 33 (35.5) | 1 (12.5) | |||
| Poorly differentiated, undifferentiated, others | 12 (14.6) | 10 (52.6) | 12 (13.8) | 10 (71.4) | 15 (16.1) | 7 (87.5) | |||
| T category | |||||||||
| pT1 + pT2 | 70 (85.4) | 14 (73.7) | .304a | 76 (87.4) | 8 (57.1) | .012a | 80 (86.0) | 4 (50.0) | .026a |
| pT3 + pT4 | 12 (14.6) | 5 (26.3) | 11 (12.6) | 6 (42.9) | 13 (14.0) | 4 (50.0) | |||
| N category | .260 | .137 | .093a | ||||||
| N0 | 31 (56.4) | 6 (40.0) | 33 (56.9) | 4 (33.3) | 36 (56.3) | 1 (16.7) | |||
| N1 + N2 | 24 (43.6) | 9 (60.0) | 25 (43.1) | 8 (66.7) | 28 (43.8) | 5 (83.3) | |||
| Pathologic stage | .116 | .045 | .010a | ||||||
| I + II | 32 (56.1) | 5 (33.3) | 34 (56.7) | 3 (25.0) | 37 (56.1) | 0 | |||
| III + IV | 25 (43.9) | 10 (66.7) | 26 (43.3) | 9 (75.0) | 29 (43.9) | 6 (100) | |||
| Growth pattern | .315 | .437 | > .99a | ||||||
| Polypoid | 45 (54.9) | 8 (42.1) | 47 (54.0) | 6 (42.9) | 49 (52.7) | 4 (50.0) | |||
| Nonpolypoid (ulcerative) | 37 (45.1) | 11 (57.9) | 40 (46.0) | 8 (57.1) | 44 (47.3) | 4 (50.0) | |||
| Lymphovascular invasion | .015 | .001 | .005a | ||||||
| No | 55 (67.1) | 7 (36.8) | 59 (67.8) | 3 (21.4) | 61 (65.6) | 1 (12.5) | |||
| Yes | 27 (32.9) | 12 (63.2) | 28 (32.2) | 11 (78.6) | 32 (34.4) | 7 (87.5) | |||
| Perineural invasion | .086 | .032a | .023a | ||||||
| No | 56 (68.3) | 9 (47.4) | 60 (69.0) | 5 (35.7) | 63 (67.7) | 2 (25.0) | |||
| Yes | 26 (31.7) | 10 (52.6) | 27 (31.0) | 9 (64.3) | 30 (32.3) | 6 (75.0) | |||
| Tumor location | .487a | .079a | .060a | ||||||
| Fundus | 30 (36.6) | 6 (31.6) | 31 (35.6) | 5 (35.7) | 35 (37.6) | 1 (12.5) | |||
| Body | 33 (40.2) | 7 (36.8) | 35 (40.2) | 5 (35.7) | 36 (38.7) | 4 (50.0) | |||
| Neck, cystic duct | 12 (14.6) | 2 (10.5) | 14 (16.1) | 0 | 14 (15.1) | 0 | |||
| More than 2 portions | 7 (8.5) | 4 (21.1) | 7 (8.0) | 4 (28.6) | 8 (8.6) | 3 (37.5) | |||
| Tumor size (cm) | .040 | .007 | .062a | ||||||
| < 2.7 | 43 (52.4) | 5 (26.3) | 46 (52.9) | 2 (14.3) | 47 (50.5) | 1 (12.5) | |||
| ≥ 2.7 | 39 (47.6) | 14 (73.7) | 41 (47.1) | 12 (85.7) | 46 (49.5) | 7 (87.5) | |||
| Complete resection | .676a | > .99a | .539a | ||||||
| Yes | 75 (91.5) | 17 (89.5) | 79 (90.8) | 13 (92.9) | 85 (91.4) | 7 (87.5) | |||
| No | 7 (8.5) | 2 (10.5) | 8 (9.2) | 1 (7.1) | 8 (8.6) | 1 (12.5) | |||
| Adjuvant chemotherapy | .720 | .344a | .233a | ||||||
| No or refuse | 57 (69.5) | 14 (73.7) | 63 (72.4) | 8 (57.1) | 67 (72.0) | 4 (50.0) | |||
| Yes | 25 (30.5) | 5 (26.3) | 24 (27.6) | 6 (42.9) | 26 (28.6) | 4 (50.0) | |||
| Gallstone | .798 | .339a | .433a | ||||||
| No | 58 (70.7) | 14 (73.7) | 60 (69.0) | 12 (85.7) | 65 (69.9) | 7 (87.5) | |||
| Yes | 24 (29.3) | 5 (26.3) | 27 (31.0) | 2 (14.3) | 28 (30.1) | 1 (12.5) | |||
| Cholecystitis | .228 | .727 | > .99 | ||||||
| No | 21 (91.3) | 2 (8.7) | 21 (91.3) | 2 (8.7) | 21 (91.3) | 2 (8.7) | |||
| Yes | 61 (78.2) | 17 (21.8) | 67 (85.9) | 11 (14.1) | 72 (92.3) | 6 (7.7) | |||
| Diabetes | .512 | .137a | .268a | ||||||
| No | 54 (65.9) | 14 (73.7) | 56 (64.4) | 12 (85.7) | 61 (65.6) | 7 (87.5) | |||
| Yes | 28 (34.1) | 5 (26.3) | 31 (35.6) | 2 (14.3) | 32 (34.4) | 1 (12.5) | |||
| Hypertension | .729 | .346 | .255a | ||||||
| No | 51 (62.2) | 11 (57.9) | 55 (63.2) | 7 (50.0) | 59 (63.4) | 3 (37.5) | |||
| Yes | 31 (37.8) | 8 (42.1) | 32 (36.8) | 7 (50.0) | 34 (36.6) | 5 (62.5) | |||
Values are presented as number (%).
Statistical analysis method: Pearson chi-square test.
PD-L1, programmed death-ligand 1; GBC, gallbladder cancer; NOS, not otherwise specified; ICPN, intracholecystic papillary neoplasm; Others, mixed adenoneuroendocrine carcinoma, signet ring cell carcinoma, mucinous carcinoma, sarcomatoid carcinoma.
Survival analysis
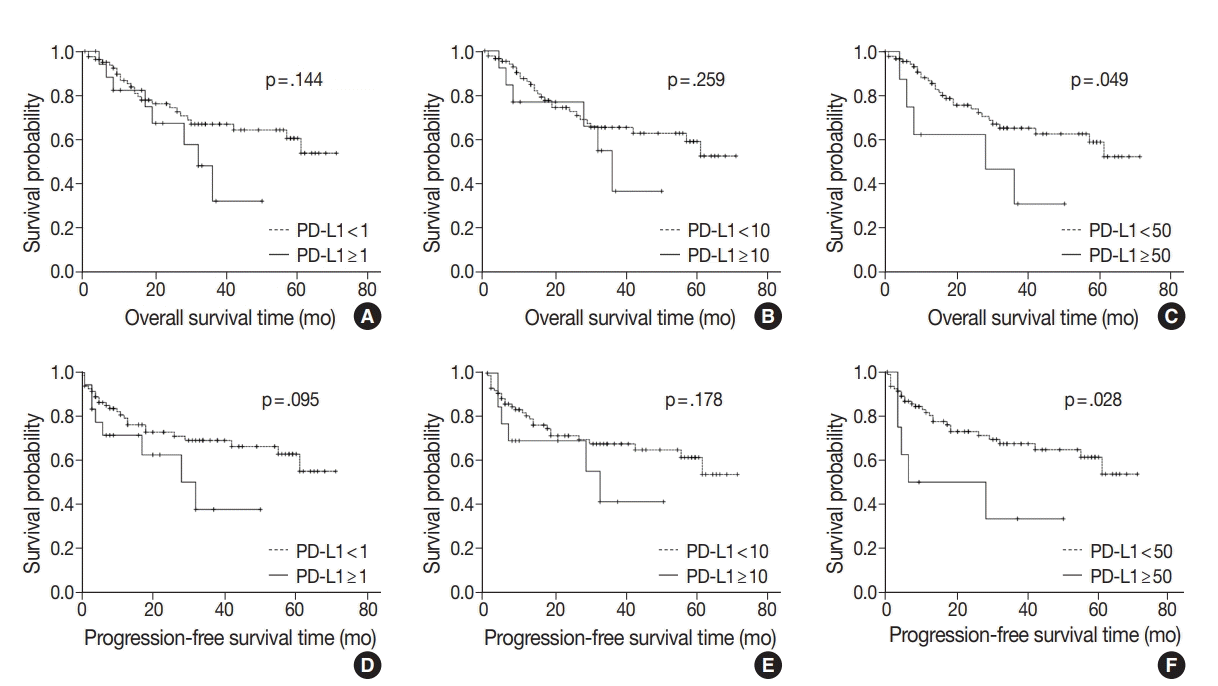 | Fig. 2.Kaplan-Meier plots for overall survival or progression-free survival of gallbladder cancer according to programmed death-ligand 1 (PD-L1) expression (A, 1% cutoff; B, 10% cutoff; C, 50% cutoff; D, 1% cutoff; E, 10% cutoff; F, 50% cutoff). |
Table 3.
Values are presented as mean ± standard error.
Statistical analysis method: survival analysis by Kaplan-Meier method and log-rank test.
OS, overall survival; PFS, progression-free survival; GBC, gallbladder cancer; NOS, not otherwise specified; ICPN, intracholecystic papillary neoplasm; others, mixed adenoneuroendocrine carcinoma, signet ring cell carcinoma, mucinous carcinoma,sarcomatoid carcinoma; PD-L1, programmed death-ligand 1.
DISCUSSION
Table 4.
| Disease | No. | Detection specimen; detection antibody | PD-L1 expression cutoff (%) | Other clinicopathological parameters associated with PD-L1 expression | Survival with PD-L1 expression | Study |
|---|---|---|---|---|---|---|
| Gallbladder cancer | 174 | FFPE tissue; anti–PD-L1 (clone SP263) | 1, 10, 50 | Significant positive association with histologic type (squamous cell carcinoma, adenosquamous cell carcinoma, undifferentiated carcinoma), histologic grade (progressed from WD to PD), nuclear grade, stage 3 and 4, TIL (0 to 3+) | OS was not associated with PD-L1 expression | Neyaz et al. [11] |
| Gallbladder adenocarcinoma | 66 | FFPE tissue; anti–PD-L1 (E1L3N) | 5 | PD-L1 positive alone was not correlated with any clinicopathological or pathological parameters except for CD8+ TIL density and worse median OS | Combination of CD8 high with negative expression of PD-L1 serves as prognostic factor for improved OS and PFS | Lin et al. [12] |
| Gastric adenocarcinoma | 240 | FFPE tissue; anti–PD-L1 (E1L3N) | 10 | Patients with poor tumor differentiation had a higher positive rate of PD-L1 expression on tumor cells | Positive PD-L1 expression on TILs had a shorter OS; However, PD-L1 expression on tumor cells was not associated with OS | Fang et al. [23] |
| Gastric cancer | 107 | FFPE tissue; anti–PDL1 (polyclonal antihuman PD-L1/CD274 antibody) | Not applicable | Positive rate of PD-L1 expression is much higher in depth of invasion, high differentiation, lymph node metastasis, and higher T category | PD-L1–positive gastric cancers were significantly associated with a poor prognosis | Qing et al. [24] |
| Esophageal cancer | 41 | Frozen tissue; anti–PD-L1 (MIH1, mouse IgG1) | 10 | Effect of PD-L1 status was more distinct in the advanced stage of tumor with lymph node metastasis and distinct metastasis | Overall survival of patients with tumors positive for both PD-L1 and PD-L2 was significantly worse than that with tumors negative for both | Ohigashi et al. [25] |
| Colorectal cancer | 143 | FFPE tissue; anti–PD-L1 (Abcam, ab58810) | Strong and moderate immunostaining intensity | PD-L1 was significantly associated with cell differentiation status and TNM stage | Positive PD-L1 expression showed a trend shorter survival time; as an independent predictor of prognosis | Shi et al. [26] |
| Lung adenocarcinoma | 163 | FFPE tissue; anti–PD-L1 (Proteintech Group Inc., Chicago, IL, USA) | 5 | PD-L1 had higher positive results in tumors with higher grade differentiation and vascular invasion | PD-L1 expression correlated with better RFS | Yang et al. [27] |
| Lung non-small cell carcinoma | 819 | FFPE tissue; anti–PD-L1 (22C3) | 50 | Lower PD-L1 positivity correlated with lower stage and squamous cell carcinoma than adenocarcinoma | Not assessed | Skov et al. [28] |
| Extrahepatic cholangiocarcinoma | 69 | FFPE tissue; anti–PD-L1 (E1L3N) | Not applicable | Significant correlations of PD-L1 expression with venous invasion and poor differentiation of the tumor were observed | PD-L1 expression was not correlated with patient OS, but combined high PDL1 expression on tumor cells and low infiltration of CD3+ TILs showed poor OS | Walter D et al. [29] |
| Hepatocellular carcinoma | 240 + additional 125 | FFPE tissue; anti–PD-L1 (eBioscience) | High vs. low | PD-L1 expression was an independent prognostic factor for tumor vascular invasion, encapsulation, and TNM stage | PD-L1–positive (high expression) patients had significantly poorer DFS and OS | Gao et al. [30] |
| Hepatocellular carcinoma | 448 | FFPE tissue; anti–PD-L1 (E1L3N) | 1, 5 | No significant difference in PD-L1 expression was detected | Survival analysis showed that 5% PD-L1 expression was significantly correlated with improved rates of OS and RFS | Chen et al. [31] |
| Uveal melanoma | 67 | FFPE tissue; anti–PD-L1 (E1L3N) | 5 | Significant association of PD-L1 expression to a decreased number of TIL | PD-L1 expression is associated with metastasis-free survival | Zoroquiain et al. [32] |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download