1. Neutra MR, Mantis NJ, Kraehenbuhl JP. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat Immunol. 2001; 2:1004–9.

2. Rubio CA, Puppa G, de Petris G, Kis L, Schmidt PT. The third pathway of colorectal carcinogenesis. J Clin Pathol. 2018; 71:7–11.

3. Elmore SA. Enhanced histopathology of mucosa-associated lymphoid tissue. Toxicol Pathol. 2006; 34:687–96.

4. Langman JM, Rowland R. The number and distribution of lymphoid follicles in the human large intestine. J Anat. 1986; 149:189–94.
5. Kealy WF. Colonic lymphoid-glandular complex (microbursa): nature and morphology. J Clin Pathol. 1976; 29:241–4.

6. Sipos F, Muzes G. Isolated lymphoid follicles in colon: switch points between inflammation and colorectal cancer? World J Gastroenterol. 2011; 17:1666–73.

7. Nascimbeni R, Villanacci V, Mariani PP, et al. Aberrant crypt foci in the human colon: frequency and histologic patterns in patients with colorectal cancer or diverticular disease. Am J Surg Pathol. 1999; 23:1256–63.
8. Fu KI, Sano Y, Kato S, et al. Incidence and localization of lymphoid follicles in early colorectal neoplasms. World J Gastroenterol. 2005; 11:6863–6.

9. Shah N, Thakkar B, Shen E, et al. Lymphocytic follicles and aggregates are a determinant of mucosal damage and duration of diarrhea. Arch Pathol Lab Med. 2013; 137:83–9.

10. Rubio CA, Asmundsson J, Silva P, Illies C, Hartman J, Kis L. Lymphoid aggregates in Crohn’s colitis and mucosal immunity. Virchows Arch. 2013; 463:637–42.

11. Shepherd NA, Griggs RK. Bowel cancer screening-generated diagnostic conundrum of the century: pseudoinvasion in sigmoid colonic polyps. Mod Pathol. 2015; 28 Suppl 1:S88–94.

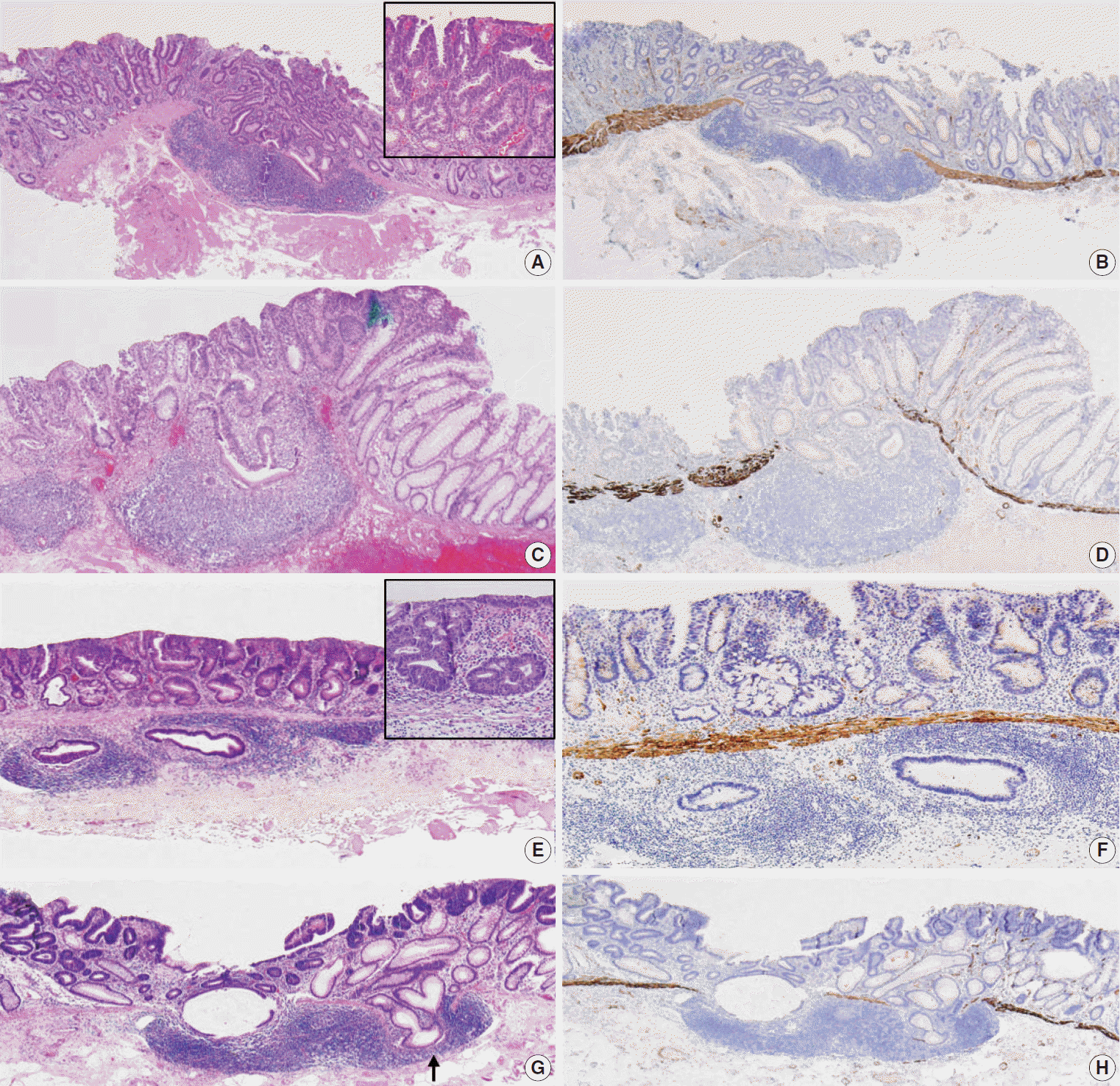
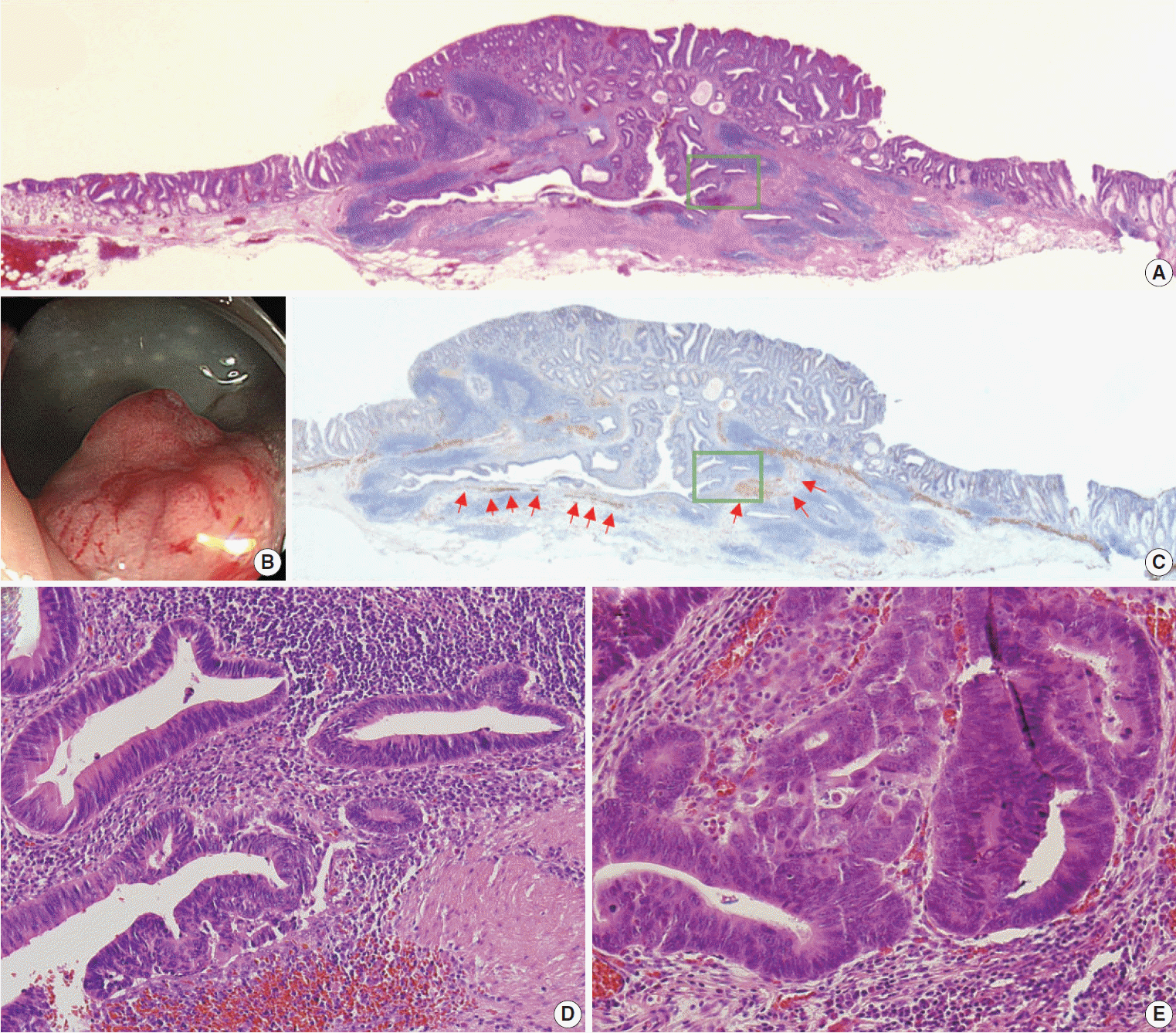
12. Lee HE, Wu TT, Chandan VS, Torbenson MS, Mounajjed T. Colonic adenomatous polyps involving submucosal lymphoglandular complexes: a diagnostic pitfall. Am J Surg Pathol. 2018; 42:1083–9.
13. McCarthy AJ, Chetty R. Gut-associated lymphoid tissue or socalled “dome” carcinoma of the colon: review. World J Gastrointest Oncol. 2019; 11:59–70.

14. Rubio CA, De Petris G, Puppa G. Gut-associated lymphoid tissue (GALT) carcinoma in ulcerative colitis. Anticancer Res. 2018; 38:919–21.

15. Zhou S, Ma Y, Chandrasoma P. Inverted lymphoglandular polyp in descending colon. Case Rep Pathol. 2015; 2015:646270.

16. Kannuna H, Rubio CA, Silverio PC, et al. DOME/GALT type adenocarcimoma of the colon: a case report, literature review and a unified phenotypic categorization. Diagn Pathol. 2015; 10:92.

17. Yamada M, Sekine S, Matsuda T. Dome-type carcinoma of the colon masquerading a submucosal tumor. Clin Gastroenterol Hepatol. 2013; 11:A30.

18. Rubio CA, Befrits R, Ericsson J. Carcinoma in gut-associated lymphoid tissue in ulcerative colitis: Case report and review of literature. World J Gastrointest Endosc. 2013; 5:293–6.

19. Yamada M, Sekine S, Matsuda T, et al. Dome-type carcinoma of the colon; a rare variant of adenocarcinoma resembling a submucosal tumor: a case report. BMC Gastroenterol. 2012; 12:21.

20. Puppa G, Molaro M. Dome-type: a distinctive variant of colonic adenocarcinoma. Case Rep Pathol. 2012; 2012:284064.

21. Coyne JD. Dome-type colorectal carcinoma: a case report and review of the literature. Colorectal Dis. 2012; 14:e360–2.

22. Rubio CA, Lindh C, Björk J, Törnblom H, Befrits R. Protruding and non-protruding colon carcinomas originating in gut-associated lymphoid tissue. Anticancer Res. 2010; 30:3019–22.
23. Stewart CJ, Hillery S, Newman N, Platell C, Ryan G. Dome-type carcinoma of the colon. Histopathology. 2008; 53:231–4.

24. Asmussen L, Pachler J, Holck S. Colorectal carcinoma with domelike phenotype: an under-recognised subset of colorectal carcinoma? J Clin Pathol. 2008; 61:482–6.

25. Rubio CA, Talbot I. Lymphoid-associated neoplasia in herniated colonic mucosa. Histopathology. 2002; 40:577–9.

26. Jass JR, Constable L, Sutherland R, et al. Adenocarcinoma of colon differentiating as dome epithelium of gut-associated lymphoid tissue. Histopathology. 2000; 36:116–20.

27. Clouston AD, Clouston DR, Jass JR. Adenocarcinoma of colon differentiating as dome epithelium of gut-associated lymphoid tissue. Histopathology. 2000; 37:567.

28. De Petris G, Lev R, Quirk DM, Ferbend PR, Butmarc JR, ElenitobaJohnson K. Lymphoepithelioma-like carcinoma of the colon in a patient with hereditary nonpolyposis colorectal cancer. Arch Pathol Lab Med. 1999; 123:720–4.

29. Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin LH, Parkin MD. International Classification of Diseases for Oncology (ICD-O). 3rd ed. Geneva: World Health Organization;2013.
30. Stintzing S, Tejpar S, Gibbs P, Thiebach L, Lenz HJ. Understanding the role of primary tumour localisation in colorectal cancer treatment and outcomes. Eur J Cancer. 2017; 84:69–80.

31. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003; 58(6 Suppl):S3–43.
32. Vleugels JLA, Hazewinkel Y, Dekker E. Morphological classifications of gastrointestinal lesions. Best Pract Res Clin Gastroenterol. 2017; 31:359–67.

33. Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005; 37:570–8.
34. Kudo S, Lambert R, Allen JI, et al. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc. 2008; 68(4 Suppl):S3–47.

35. Schlemper RJ, Hirata I, Dixon MF. The macroscopic classification of early neoplasia of the digestive tract. Endoscopy. 2002; 34:163–8.

36. Lokuhetty D, White VA, Watanabe R, Cree IA; Organizacion Mundial de la Salud; International Agency for Research on Cancer. WHO classification of tumours. Vol. 1. Digestive system tumours. 5th ed. Lyon: IARC Press;2019.
37. Fenoglio-Preiser CM, Noffsinger AE, Stemmermann GN, Lantz PE, Isaacson PG. Gastrointestinal pathology: an atlas and text. 3rd ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins;2008. p. 926–31.
38. Muto T, Bussey HJ, Morson BC. Pseudo-carcinomatous invasion in adenomatous polyps of the colon and rectum. J Clin Pathol. 1973; 26:25–31.

39. Dirschmid K, Kiesler J, Mathis G, Beller S, Stoss F, Schobel B. Epithelial misplacement after biopsy of colorectal adenomas. Am J Surg Pathol. 1993; 17:1262–5.

40. Yantiss RK, Bosenberg MW, Antonioli DA, Odze RD. Utility of MMP-1, p53, E-cadherin, and collagen IV immunohistochemical stains in the differential diagnosis of adenomas with misplaced epithelium versus adenomas with invasive adenocarcinoma. Am J Surg Pathol. 2002; 26:206–15.

41. Tanizawa T, Seki T, Nakano M, Kamiyama R. Pseudoinvasion of the colorectal polypoid tumors: serial section study of problematic cases. Pathol Int. 2003; 53:584–90.

42. Molavi D, Argani P. Distinguishing benign dissecting mucin (stromal mucin pools) from invasive mucinous carcinoma. Adv Anat Pathol. 2008; 15:1–17.

43. Loughrey MB, Shepherd NA. The pathology of bowel cancer screening. Histopathology. 2015; 66:66–77.

44. Panarelli NC, Somarathna T, Samowitz WS, et al. Diagnostic challenges caused by endoscopic biopsy of colonic polyps: a systematic evaluation of epithelial misplacement with review of problematic polyps from the bowel cancer screening program, United Kingdom. Am J Surg Pathol. 2016; 40:1075–83.
45. Ferreira da Silva MJ, Pinho R, Wen X, Tente D, Leite S, Carvalho J. Adenoma with pseudoinvasion: s crucial differential diagnosis for invasive adenocarcinoma. Gastroenterol Hepatol. 2017; 40:96–8.
46. Chang HJ, Park CK, Kim WH, et al. A standardized pathology report for colorectal cancer. Korean J Pathol. 2006; 40:193–203.
47. Rubio CA. Ectopic colonic mucosa in ulcerative colitis and in Crohn’s disease of the colon. Dis Colon Rectum. 1984; 27:182–6.

48. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013; 310:2191–4.
49. Nauss KM, Locniskar M, Pavlina T, Newberne PM. Morphology and distribution of 1,2-dimethylhydrazine dihydrochloride-induced colon tumors and their relationship to gut-associated lymphoid tissue in the rat. J Natl Cancer Inst. 1984; 73:915–24.
50. Carter JW, Lancaster HK, Hardman WE, Cameron IL. Distribution of intestine-associated lymphoid tissue, aberrant crypt foci, and tumors in the large bowel of 1,2-dimethylhydrazine-treated mice. Cancer Res. 1994; 54:4304–7.
51. Hardman WE, Cameron IL. Colonic crypts located over lymphoid nodules of 1,2-dimethylhydrazine-treated rats are hyperplastic and at high risk of forming adenocarcinomas. Carcinogenesis. 1994; 15:2353–61.

52. Rubio CA, Shetye J, Jaramillo E. Non-polypoid adenomas of the colon are associated with subjacent lymphoid nodules: an experimental study in rats. Scand J Gastroenterol. 1999; 34:504–8.
53. Rubio CA. The histogenesis of the third pathway of colonic carcinogenesis in rats. Anticancer Res. 2017; 37:1039–42.
54. Dukes C, Bussey HJ. The number of lymphoid follicles of the human large intestine. J Pathol Bacteriol. 1926; 29:111–6.

55. O’Leary AD, Sweeney EC. Lymphoglandular complexes of the colon: structure and distribution. Histopathology. 1986; 10:267–83.

56. Rubio CA, Schmidt PT. Gut-associated lymphoid tissue (GALT) carcinoma or dome carcinoma? Anticancer Res. 2016; 36:5385–7.

57. Greene FL. Epithelial misplacement in adenomatous polyps of the colon and rectum. Cancer. 1974; 33:206–17.

58. Fenoglio-Preiser CM, Lantz P, Listrom M, Noffsinger A, Riker F, Stemmermann G. Gastrointestinal pathology: an atlas and text. 2nd ed. Philadelphia: Lippincott-Raven;1999.
59. Schlemper RJ, Itabashi M, Kato Y, et al. Differences in the diagnostic criteria used by Japanese and Western pathologists to diagnose colorectal carcinoma. Cancer. 1998; 82:60–9.

60. Risio M. The natural history of pT1 colorectal cancer. Front Oncol. 2012; 2:22.

61. Quirke P, Risio M, Lambert R, von Karsa L, Vieth M. Quality assurance in pathology in colorectal cancer screening and diagnosis-European recommendations. Virchows Arch. 2011; 458:1–19.

62. Lewin MR, Fenton H, Burkart AL, Sheridan T, Abu-Alfa AK, Montgomery EA. Poorly differentiated colorectal carcinoma with invasion restricted to lamina propria (intramucosal carcinoma): a follow-up study of 15 cases. Am J Surg Pathol. 2007; 31:1882–6.

63. Kenney BC, Jain D. Identification of lymphatics within the colonic lamina propria in inflammation and neoplasia using the monoclonal antibody D2-40. Yale J Biol Med. 2008; 81:103–13.
64. Fenoglio CM, Kaye GI, Lane N. Distribution of human colonic lymphatics in normal, hyperplastic, and adenomatous tissue. Its relationship to metastasis from small carcinomas in pedunculated adenomas, with two case reports. Gastroenterology. 1973; 64:51–66.
65. Hashimoto H, Horiuchi H, Kurata A, et al. Intramucosal colorectal carcinoma with lymphovascular invasion: clinicopathological characteristics of nine cases. Histopathology. 2019; 74:1055–66.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download