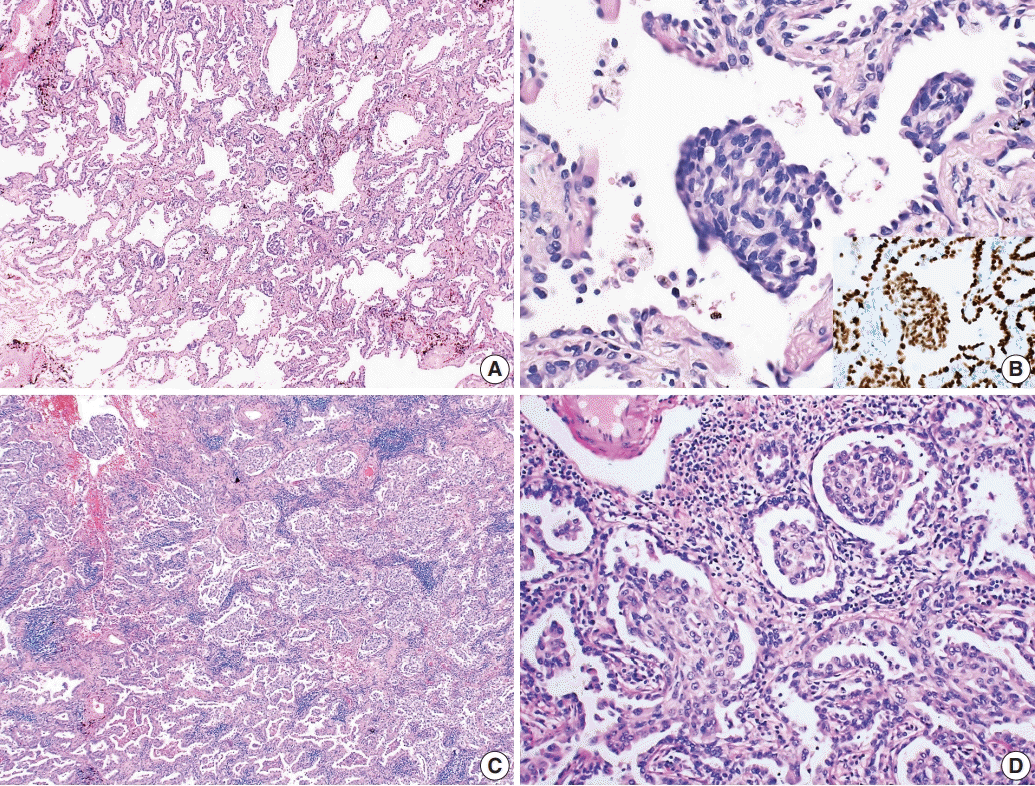
Morules, or morule-like features, can be identified in both benign and malignant lesions in various organs [
1]. A subset of pulmonary adenocarcinomas show morule-like features, particularly papillary-predominant adenocarcinoma [
2]. Morules can also be identified in adenocarcinoma in situ (AIS); previously known as bronchioloalveolar carcinoma [
3]. One case in this report showed morule-like features in AIS and the other case showed these features in invasive adenocarcinoma. Using next-generation sequencing (NGS), we attempted to identify the molecular characteristics of these rare morular features in the two cases.
DISCUSSION
Adenocarcinoma with morule-like features is a rare variant of lung adenocarcinoma, accounting for 1.9% of all adenocarcinomas [
2]. To date, 26 cases of lung adenocarcinoma with morule-like features have been reported [
1-
5] that were reported as papillary (15 cases), micropapillary (3 cases), acinar (3 cases), and solid-predominant adenocarcinoma (3 cases). The remaining two cases were adenocarcinoma with a lepidic pattern [
2], and bronchioloalveolar carcinoma (AIS) [
3].
Histologically, the cells comprising the morular feature were spindle shaped and distinct from the other cancer cells. There are several possibilities to explain the pathophysiology of this unusual morular component. Traditionally, morules have been reported in pulmonary blastoma and well-differentiated fetal adenocarcinoma, and are regarded as non-epithelial cell clusters showing neuronal differentiation [
1,
6]. However, morular cells in the cases reported here were positive for pulmonary epithelial markers, including CK and TTF-1, but negative for neuroendocrine markers, consistent with several other reports [
1-
3,
5]. As such, they can be considered a complex glandular component, or epithelial cell nodules. Therefore, the presence of a morular component in small biopsy specimens does not, by itself, indicate pulmonary blastoma and immunohistochemistry can help to determine the likelihood of epithelial carcinoma. Furthermore, although the morular cells are spindle shaped, Moran et al. [
5] reported that negative results for muscle markers and human melanoma black-45 reduce the probability of smooth muscle tumor and lymphangioleiomyomatosis.
Fornelli et al. [
3] reported the first case of AIS with morule-like features as a non-invasive tumor because both architectural complexity and stromal desmoplasia were not apparent. Fornelli’s case is the only report currently available in the literature, making our reported cases the second study of AIS showing morule-like features. Tsuta et al. [
2] found that lung adenocarcinomas with morule-like features have lower 5-year overall survival rates, suggesting that morule-like features are an invasive component similar to an aggressive histologic micropapillary pattern. On the other hand, one report suggested that the morular feature itself might not have prognostic significance [
7], and the invasion criteria for the pulmonary adenocarcinoma used in World Health Organization 2015 classification of tumors of the lung, pleura, thymus, and heart does not include morular features. Therefore, if there is no other evidence of stromal invasion, AIS with morular features should be evaluated separately from the invasive adenocarcinoma. This case of AIS did not show any evidence of recurrence or metastasis at 1-year follow-up; however, further studies of AIS with morule-like features are needed.
Depending on the observer, morular like components filling the intra-alveolar space can be considered as an invasive lesion, or an airspace invasion forming a solid nest. From that point of view, case 1 described here can be diagnosed as lepidic predominant adenocarcinoma, as morular portion size is 0.7 cm in maximum diameter. However, further research will be needed as some argue that there is no prognostic significance [
7].
The molecular characteristics of the morular component are not fully understood. However, several studies have revealed that this feature is associated with
EGFR mutations. In a previous report that analyzed two common
EGFR mutations, including deletions in exon 19 and a point mutation at codon 858 in exon 21 (L858R), the presence of morule-like components correlated with
EGFR mutations, particularly deletions, and was reported to be an independent predictive factor of
EGFR mutation status [
2]. Another report by Tajima and Koda [
4] found that morule-like features were associated with a deletion mutation at exon 19 in
EGFR. Ours is the first report in which NGS was used to identify the molecular characteristics of morule-like features in pulmonary adenocarcinoma. Our results revealed unusual
EGFR mutations that have not been previously reported in pulmonary adenocarcinoma with morule-like features. The rare mutation in
EGFR exon 19 and the insertion mutation in
EGFR exon 20 can be associated with drug resistance to EGFR tyrosine kinase inhibitors [
8-
10], implying the importance of identifying morule-like features in pulmonary adenocarcinoma.
Morule-like components are a rare histologic feature of pulmonary adenocarcinoma, which may be associated with rare EGFR mutations, such as the L747S missense mutation of exon 19 or an insertion mutation in exon 20. The presence of morule-like features without definite invasive components led to the diagnosis of AIS in one of these cases, as it is not yet clear whether this feature needs to be considered as an invasive component. Additional studies are needed to clarify the significance of this rare histologic variant in pulmonary adenocarcinoma.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download