1. Scully C, Bagan J. Oral squamous cell carcinoma: overview of current understanding of aetiopathogenesis and clinical implications. Oral Dis. 2009; 15:388–99.

2. Ng JH, Iyer NG, Tan MH, Edgren G. Changing epidemiology of oral squamous cell carcinoma of the tongue: a global study. Head Neck. 2017; 39:297–304.

3. Patel SC, Carpenter WR, Tyree S, et al. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol. 2011; 29:1488–94.

4. Harris SL, Kimple RJ, Hayes DN, Couch ME, Rosenman JG. Never-smokers, never-drinkers: unique clinical subgroup of young patients with head and neck squamous cell cancers. Head Neck. 2010; 32:499–503.

5. Choi SW, Moon EK, Park JY, et al. Trends in the incidence of and survival rates for oral cavity cancer in the Korean population. Oral Dis. 2014; 20:773–9.

6. Blanchard P, Belkhir F, Temam S, et al. Outcomes and prognostic factors for squamous cell carcinoma of the oral tongue in young adults: a single-institution case-matched analysis. Eur Arch Otorhinolaryngol. 2017; 274:1683–90.

7. Goepfert RP, Kezirian EJ, Wang SJ. Oral tongue squamous cell carcinoma in young women: a matched comparison-do outcomes justify treatment intensity? ISRN Otolaryngol. 2014; 2014:529395.

8. Majchrzak E, Szybiak B, Wegner A, et al. Oral cavity and oropharyngeal squamous cell carcinoma in young adults: a review of the literature. Radiol Oncol. 2014; 48:1–10.

9. van Monsjou HS, Wreesmann VB, van den Brekel MW, Balm AJ. Head and neck squamous cell carcinoma in young patients. Oral Oncol. 2013; 49:1097–102.

10. Pickering CR, Zhang J, Neskey DM, et al. Squamous cell carcinoma of the oral tongue in young non-smokers is genomically similar to tumors in older smokers. Clin Cancer Res. 2014; 20:3842–8.

11. Santos HB, dos Santos TK, Paz AR, et al. Clinical findings and risk factors to oral squamous cell carcinoma in young patients: a 12-year retrospective analysis. Med Oral Patol Oral Cir Bucal. 2016; 21:e151–6.

12. Lingen MW, Chang KW, McMurray SJ, et al. Overexpression of p53 in squamous cell carcinoma of the tongue in young patients with no known risk factors is not associated with mutations in exons 5-9. Head Neck. 2000; 22:328–35.

13. Grimm M, Biegner T, Teriete P, et al. Estrogen and progesterone hormone receptor expression in oral cavity cancer. Med Oral Patol Oral Cir Bucal. 2016; 21:e554–8.

14. Colella G, Izzo G, Carinci F, et al. Expression of sexual hormones receptors in oral squamous cell carcinoma. Int J Immunopathol Pharmacol. 2011; 24(2 Suppl):129–32.

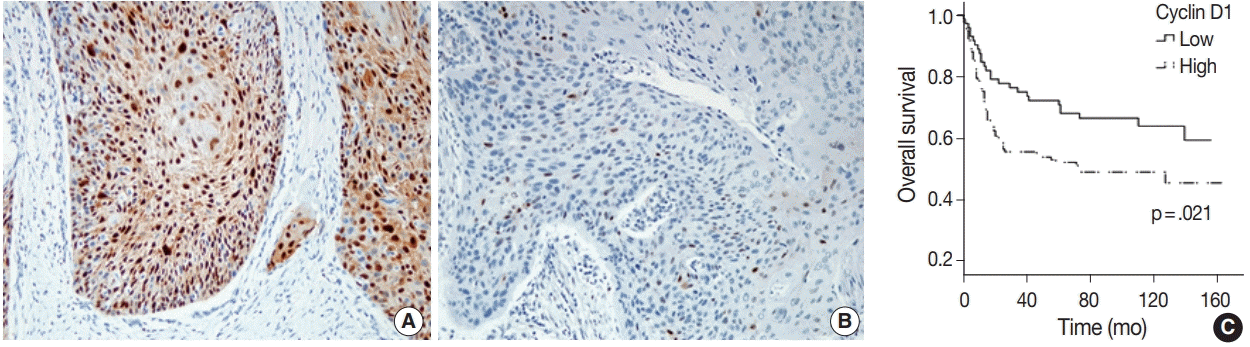
15. Kaminagakura E, Werneck da Cunha I, Soares FA, Nishimoto IN, Kowalski LP. CCND1 amplification and protein overexpression in oral squamous cell carcinoma of young patients. Head Neck. 2011; 33:1413–9.

16. Lang J, Song X, Cheng J, Zhao S, Fan J. Association of GSTP1 Il-e105Val polymorphism and risk of head and neck cancers: a metaanalysis of 28 case-control studies. PLoS One. 2012; 7:e48132.

17. Soares PO, Maluf Cury P, Mendoza Lopez RV, et al. GTSP1 expression in non-smoker and non-drinker patients with squamous cell carcinoma of the head and neck. PLoS One. 2017; 12:e0182600.

18. Yanamoto S, Kawasaki G, Yoshitomi I, Mizuno A. p53, mdm2, and p21 expression in oral squamous cell carcinomas: relationship with clinicopathologic factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002; 94:593–600.

19. Mineta H, Miura K, Takebayashi S, et al. Cyclin D1 overexpression correlates with poor prognosis in patients with tongue squamous cell carcinoma. Oral Oncol. 2000; 36:194–8.

20. Harris SL, Thorne LB, Seaman WT, Hayes DN, Couch ME, Kimple RJ. Association of p16(INK4a) overexpression with improved outcomes in young patients with squamous cell cancers of the oral tongue. Head Neck. 2011; 33:1622–7.

21. Karen-Ng LP, Marhazlinda J, Rahman ZA, et al. Combined effects of isothiocyanate intake, glutathione S-transferase polymorphisms and risk habits for age of oral squamous cell carcinoma development. Asian Pac J Cancer Prev. 2011; 12:1161–6.
22. Koenigs MB, Lefranc-Torres A, Bonilla-Velez J, et al. Association of estrogen receptor alpha expression with survival in oropharyngeal cancer following chemoradiation therapy. J Natl Cancer Inst. 2019; 111:933–42.

23. Mohamed H, Aro K, Jouhi L, et al. Expression of hormone receptors in oropharyngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2018; 275:1289–300.

24. Morse DE, Katz RV, Pendrys DG, et al. Smoking and drinking in relation to oral epithelial dysplasia. Cancer Epidemiol Biomarkers Prev. 1996; 5:769–77.
25. Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988; 48:3282–7.
26. Morse DE, Psoter WJ, Cleveland D, et al. Smoking and drinking in relation to oral cancer and oral epithelial dysplasia. Cancer Causes Control. 2007; 18:919–29.

27. Amin MB, Edge S, Greene F, et al. AJCC cancer staging manual. 8th. New York: Springer;2017.
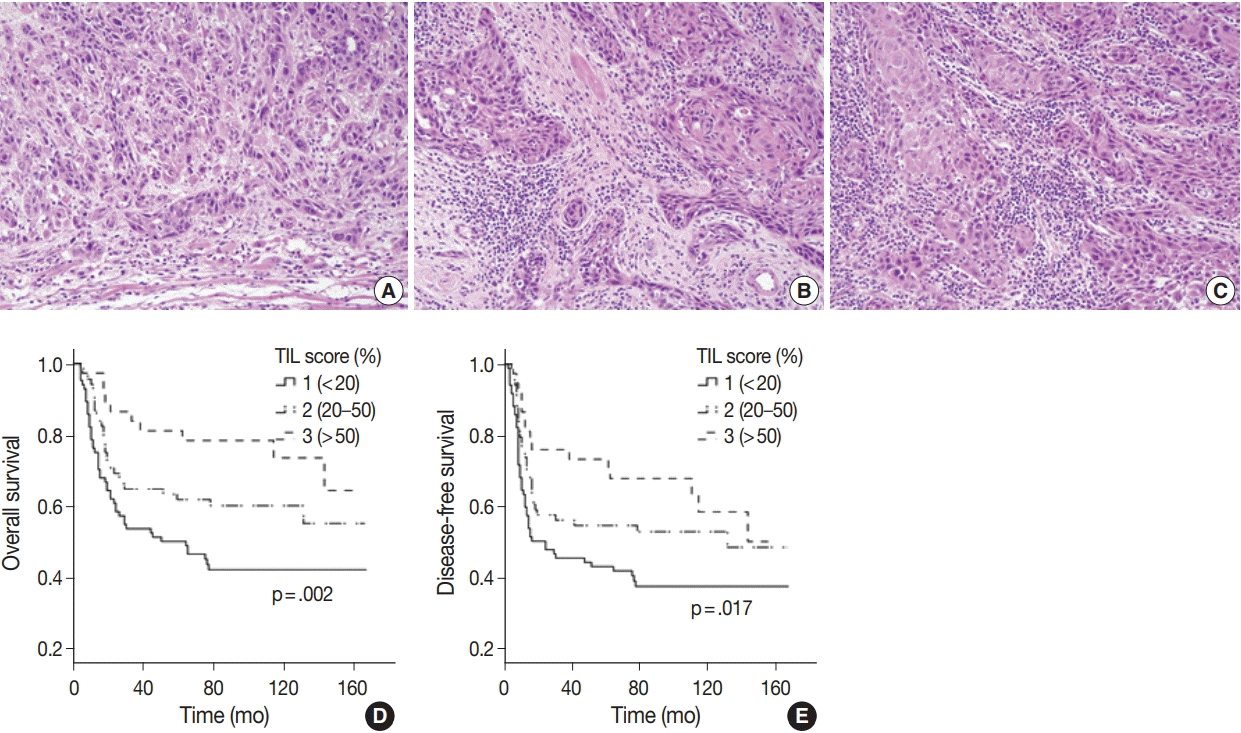
28. Hendry S, Salgado R, Gevaert T, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the International Immunooncology Biomarkers Working Group: Part 1: assessing the host immune response, TILs in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Adv Anat Pathol. 2017; 24:235–51.

29. Hendry S, Salgado R, Gevaert T, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol. 2017; 24:311–35.

30. Ofner D, Maier H, Riedmann B, et al. Immunohistochemically detectable p53 and mdm-2 oncoprotein expression in colorectal carcinoma: prognostic significance. Clin Mol Pathol. 1995; 48:M12–6.

31. Klaes R, Friedrich T, Spitkovsky D, et al. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001; 92:276–84.

32. Zhang YY, Wang DC, Su JZ, Jia LF, Peng X, Yu GY. Clinicopathological characteristics and outcomes of squamous cell carcinoma of the tongue in different age groups. Head Neck. 2017; 39:2276–82.

33. Dediol E, Sabol I, Virag M, Grce M, Muller D, Manojlovic S. HPV prevalence and p16INKa overexpression in non-smoking non-drinking oral cavity cancer patients. Oral Dis. 2016; 22:517–22.

34. Dos Santos Costa SF, Brennan PA, Gomez RS, et al. Molecular basis of oral squamous cell carcinoma in young patients: is it any different from older patients? J Oral Pathol Med. 2018; 47:541–6.

35. Sun Q, Fang Q, Guo S. A comparison of oral squamous cell carcinoma between young and old patients in a single medical center in China. Int J Clin Exp Med. 2015; 8:12418–23.
36. Chang YL, Hsu YK, Wu TF, et al. Regulation of estrogen receptor alpha function in oral squamous cell carcinoma cells by FAK signaling. Endocr Relat Cancer. 2014; 21:555–65.
37. Wu TF, Luo FJ, Chang YL, et al. The oncogenic role of androgen receptors in promoting the growth of oral squamous cell carcinoma cells. Oral Dis. 2015; 21:320–7.

38. Mutallip M, Nohata N, Hanazawa T, et al. Glutathione S-transferase P1 (GSTP1) suppresses cell apoptosis and its regulation by miR133alpha in head and neck squamous cell carcinoma (HNSCC). Int J Mol Med. 2011; 27:345–52.

39. Zhao Y, Yu D, Li H, et al. Cyclin D1 overexpression is associated with poor clinicopathological outcome and survival in oral squamous cell carcinoma in Asian populations: insights from a metaanalysis. PLoS One. 2014; 9:e93210.

40. Brandwein-Gensler M, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005; 29:167–78.
41. Cho YA, Yoon HJ, Lee JI, Hong SP, Hong SD. Relationship between the expressions of PD-L1 and tumor-infiltrating lymphocytes in oral squamous cell carcinoma. Oral Oncol. 2011; 47:1148–53.

42. Chang TS, Chang CM, Ho HC, et al. Impact of young age on the prognosis for oral cancer: a population-based study in Taiwan. PLoS One. 2013; 8:e75855.