1. Horn LC, Meinel A, Handzel R, Einenkel J. Histopathology of endometrial hyperplasia and endometrial carcinoma: an update. Ann Diagn Pathol. 2007; 11:297–311.
2. Banas T, Pitynski K, Okon K, Winiarska A. Non-endometrioid and high-grade endometrioid endometrial cancers show DNA fragmentation factor 40 (DFF40) and B-cell lymphoma 2 protein (BCL2) underexpression, which predicts disease-free and overall survival, but not DNA fragmentation factor 45 (DFF45) underexpression. BMC Cancer. 2018; 18:418.

3. Reeves KW, Carter GC, Rodabough RJ, et al. Obesity in relation to endometrial cancer risk and disease characteristics in the Women’s Health Initiative. Gynecol Oncol. 2011; 121:376–82.

4. SGO Clinical Practice Endometrial Cancer Working Group, Burke WM, Orr J, et al. Endometrial cancer: a review and current management strategies: part II. Gynecol Oncol. 2014; 134:393–402.

5. Murray SK, Young RH, Scully RE. Unusual epithelial and stromal changes in myoinvasive endometrioid adenocarcinoma: a study of their frequency, associated diagnostic problems, and prognostic significance. Int J Gynecol Pathol. 2003; 22:324–33.

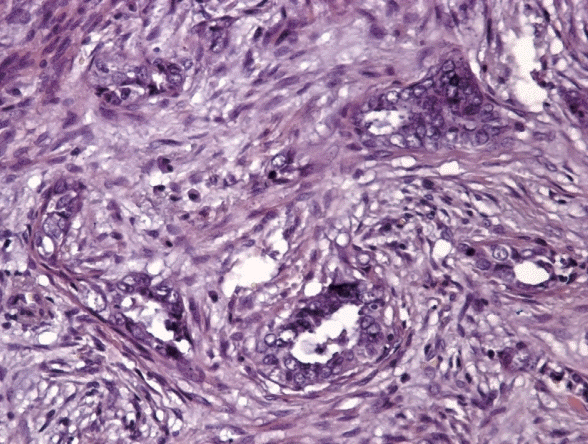
6. Sanci M, Güngördük K, Gülseren V, et al. MELF pattern for predicting lymph node involvement and survival in grade I-II endometrioid-type endometrial cancer. Int J Gynecol Pathol. 2018; 37:17–21.

7. Kihara A, Yoshida H, Watanabe R, et al. Clinicopathologic association and prognostic value of microcystic, elongated, and fragmented (MELF) pattern in endometrial endometrioid carcinoma. Am J Surg Pathol. 2017; 41:896–905.

8. Pelletier MP, Trinh VQ, Stephenson P, et al. Microcystic, elongated, and fragmented pattern invasion is mainly associated with isolated tumor cell pattern metastases in International Federation of Gynecology and Obstetrics grade I endometrioid endometrial cancer. Hum Pathol. 2017; 62:33–9.

9. Stewart CJ, Brennan BA, Leung YC, Little L. MELF pattern invasion in endometrial carcinoma: association with low grade, myoinvasive endometrioid tumours, focal mucinous differentiation and vascular invasion. Pathology. 2009; 41:454–9.

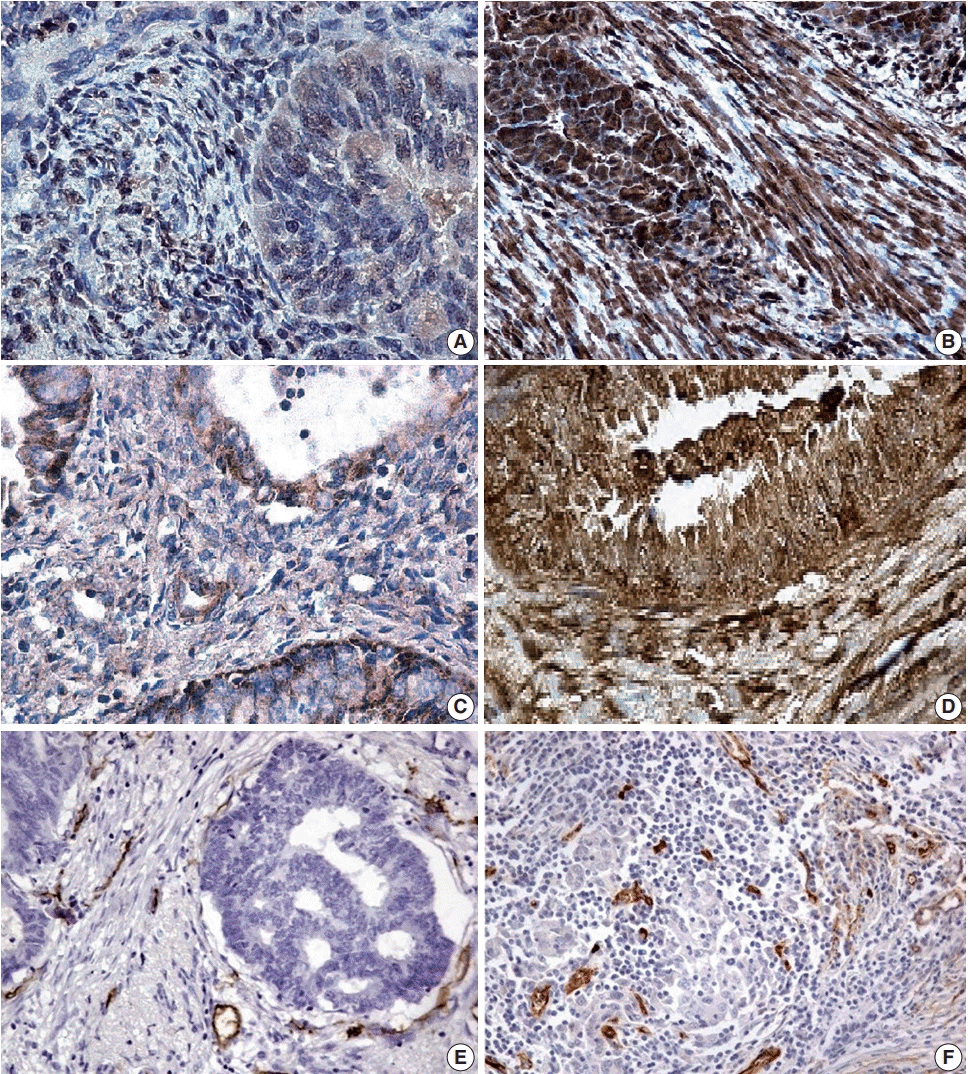
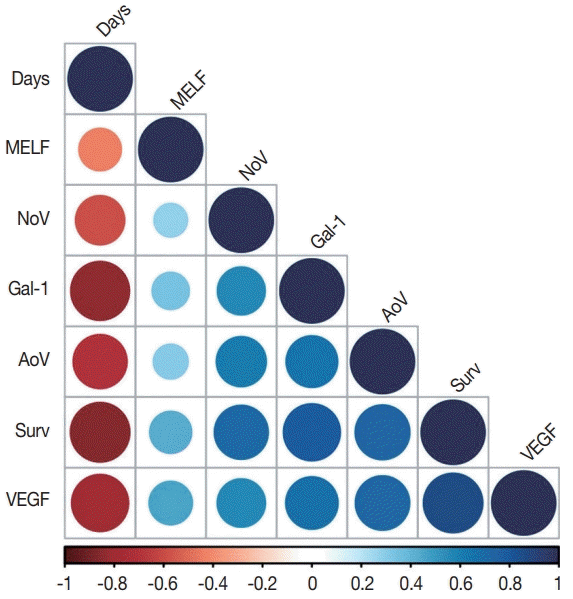
10. Zinovkin DA, Pranjol MZI, Petrenyov DR, Nadyrov EA, Savchenko OG. The potential roles of MELF-pattern, microvessel density, and VEGF expression in survival of patients with endometrioid endometrial carcinoma: a morphometrical and immunohistochemical analysis of 100 cases. J Pathol Transl Med. 2017; 51:456–62.

11. Zinovkin D, Pranjol MZ. Tumor-infiltrated lymphocytes, macrophages, and dendritic cells in endometrioid adenocarcinoma of corpus uteri as potential prognostic factors: an immunohistochemical study. Int J Gynecol Cancer. 2016; 26:1207–12.

12. Zhu X, Wang K, Zhang K, et al. Galectin-1 knockdown in carcinomaassociated fibroblasts inhibits migration and invasion of human MDA-MB-231 breast cancer cells by modulating MMP-9 expression. Acta Biochim Biophys Sin (Shanghai). 2016; 48:462–7.

13. Jeschke U, Walzel H, Mylonas I, et al. The human endometrium expresses the glycoprotein mucin-1 and shows positive correlation for Thomsen-Friedenreich epitope expression and galectin-1 binding. J Histochem Cytochem. 2009; 57:871–81.

14. Sandberg TP, Oosting J, van Pelt GW, Mesker WE, Tollenaar R, Morreau H. Molecular profiling of colorectal tumors stratified by the histological tumor-stroma ratio: increased expression of galectin-1 in tumors with high stromal content. Oncotarget. 2018; 9:31502–15.
15. Zinovkin DA, Pranjol MZ, Bilsky IA, Zmushko VA. Tumor-associated T-lymphocytes and macrophages are decreased in endometrioid endometrial carcinoma with MELF-pattern stromal changes. Cancer Microenviron. 2018; 11:107–14.

16. Albini A, Bruno A, Noonan DM, Mortara L. Contribution to tumor angiogenesis from innate immune cells within the tumor microenvironment: implications for immunotherapy. Front Immunol. 2018; 9:527.

17. Melincovici CS, Bos¸ca AB, S¸us¸man S, et al. Vascular endothelial growth factor (VEGF): key factor in normal and pathological angiogenesis. Rom J Morphol Embryol. 2018; 59:455–67.
18. Tao L, Huang G, Song H, Chen Y, Chen L. Cancer associated fibroblasts: an essential role in the tumor microenvironment. Oncol Lett. 2017; 14:2611–20.

19. Li YL, Zhao H, Ren XB. Relationship of VEGF/VEGFR with immune and cancer cells: staggering or forward? Cancer Biol Med. 2016; 13:206–14.

20. Miyata Y, Sakai H. Reconsideration of the clinical and histopathological significance of angiogenesis in prostate cancer: Usefulness and limitations of microvessel density measurement. Int J Urol. 2015; 22:806–15.

21. Jilaveanu LB, Puligandla M, Weiss SA, et al. Tumor microvessel density as a prognostic marker in high-risk renal cell carcinoma patients treated on ECOG-ACRIN E2805. Clin Cancer Res. 2018; 24:217–23.

22. Hu X, Liu H, Ye M, Zhu X. Prognostic value of microvessel density in cervical cancer. Cancer Cell Int. 2018; 18:152.

23. Mezheyeuski A, Nerovnya A, Bich T, Tur G, Ostman A, Portyanko A. Inter- and intra-tumoral relationships between vasculature characteristics, GLUT1 and budding in colorectal carcinoma. Histol Histopathol. 2015; 30:1203–11.
24. Joehlin-Price AS, McHugh KE, Stephens JA, et al. The microcystic, elongated, and fragmented (MELF) pattern of invasion: a single institution report of 464 consecutive FIGO grade 1 endometrial endometrioid adenocarcinomas. Am J Surg Pathol. 2017; 41:49–55.
25. Kamili NA, Arthur CM, Gerner-Smidt C, et al. Key regulators of galectin-glycan interactions. Proteomics. 2016; 16:3111–25.

26. Chen J, Tang D, Wang S, et al. High expressions of galectin-1 and VEGF are associated with poor prognosis in gastric cancer patients. Tumour Biol. 2014; 35:2513–9.

27. Wu R, Wu T, Wang K, et al. Prognostic significance of galectin-1 expression in patients with cancer: a meta-analysis. Cancer Cell Int. 2018; 18:108.

28. Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013; 13:871–82.

29. Wang JZ, Xiong YJ, Man GC, Chen XY, Kwong J, Wang CC. Clinicopathological and prognostic significance of blood microvessel density in endometrial cancer: a meta-analysis and subgroup analysis. Arch Gynecol Obstet. 2018; 297:731–40.

30. Prodromidou A, Vorgias G, Bakogiannis K, Kalinoglou N, Iavazzo C. MELF pattern of myometrial invasion and role in possible endometrial cancer diagnostic pathway: a systematic review of the literature. Eur J Obstet Gynecol Reprod Biol. 2018; 230:147–52.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download