Abstract
We present the case of a 71-year-old man who was diagnosed with amoebic encephalitis caused by Balamuthia mandrillaris. He had rheumatic arthritis for 30 years and had undergone continuous treatment with immunosuppressants. First, he complained of partial spasm from the left thigh to the left upper limb. Magnetic resonance imaging revealed multifocal enhancing nodules in the cortical and subcortical area of both cerebral hemispheres, which were suggestive of brain metastases. However, the patient developed fever with stuporous mentality and an open biopsy was performed immediately. Microscopically, numerous amoebic trophozoites, measuring 20 to 25 µm in size, with nuclei containing one to four nucleoli and some scattered cysts having a double-layered wall were noted in the background of hemorrhagic necrosis. Based on the microscopic findings, amoebic encephalitis caused by Balamuthia mandrillaris was diagnosed. The patient died on the 10th day after being admitted at the hospital. The diagnosis of amoebic encephalitis in the early stage is difficult for clinicians. Moreover, most cases undergo rapid deterioration, resulting in fatal consequences. In this report, we present the first case of B. mandrillaris amoebic encephalitis with fatal progression in a Korean patient.
Go to : 
Although amoebic encephalitis is a rare disease, it has a very high mortality rate. There are two distinct forms of representation of this disease. One is the granulomatous amoebic encephalitis caused by Balamuthia mandrillaris or Acanthamoeba species, which progresses chronically or subacutely, during a period of two weeks to 2 years. The other is the primary amoebic meningoencephalitis caused by Naegleria fowleri, which progresses rapidly and fulminantly, resulting in death within a week or less [1].
Unlike the other amoeba species, B. mandrillaris affects not only immunocompromised but also immunocompetent people, especially the young and the elderly [2]. The histopathologic findings showed a wide spectrum of features from acute and subacute to granulomatous inflammation. In addition, the mortality rate is known to be 98% [3]. Since 1991, B. mandrillaris encephalitis has been reported in more than 200 cases in the world and has predominantly occurred in South America and the United States. However, in East Asia, only nine cases have been reported in Japan [4] and the present patient is the first case of B. mandrillaris encephalitis in the Republic of Korea.
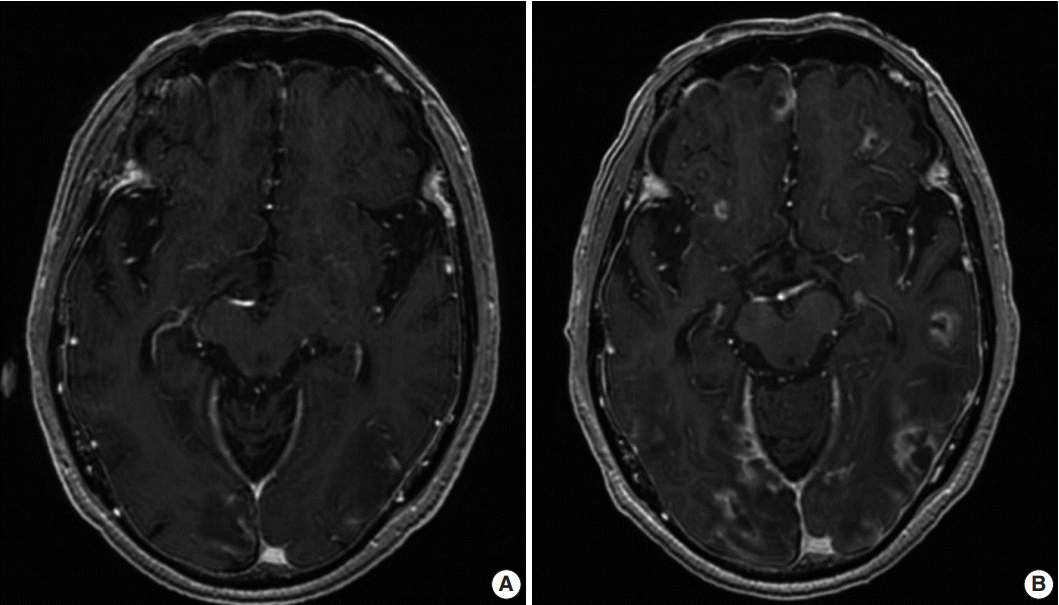
A 71-year-old Korean man presented with partial spasm from the left thigh to the left upper limb for a day without any infectious symptoms at that time. He had had rheumatic arthritis for 30 years and had been continuously treated with immunosuppressants such as steroids, methotrexate, and nonsteroidal antiinflammatory drugs. Initial magnetic resonance imaging (MRI) of the brain revealed several ring-enhancing nodules measuring up to 3.7×3.2×1.8 cm in size in both cerebral hemispheres, and brain metastasis of unknown origin was suspected (Fig. 1A). The initial blood laboratory test revealed an increased C-reactive protein (CRP) level (7.89 mg/dL) and a normal white blood cell count. The cerebrospinal fluid (CSF) study revealed that the glucose level was within the normal range of 60 mg/dL and that the protein level was elevated at 331.8 mg/dL. A week after, the patient complained of fever and stuporous mentality; a second brain MRI performed at that time revealed a number of nodules that were larger in size (Fig. 1B) compared with the initial MRI.
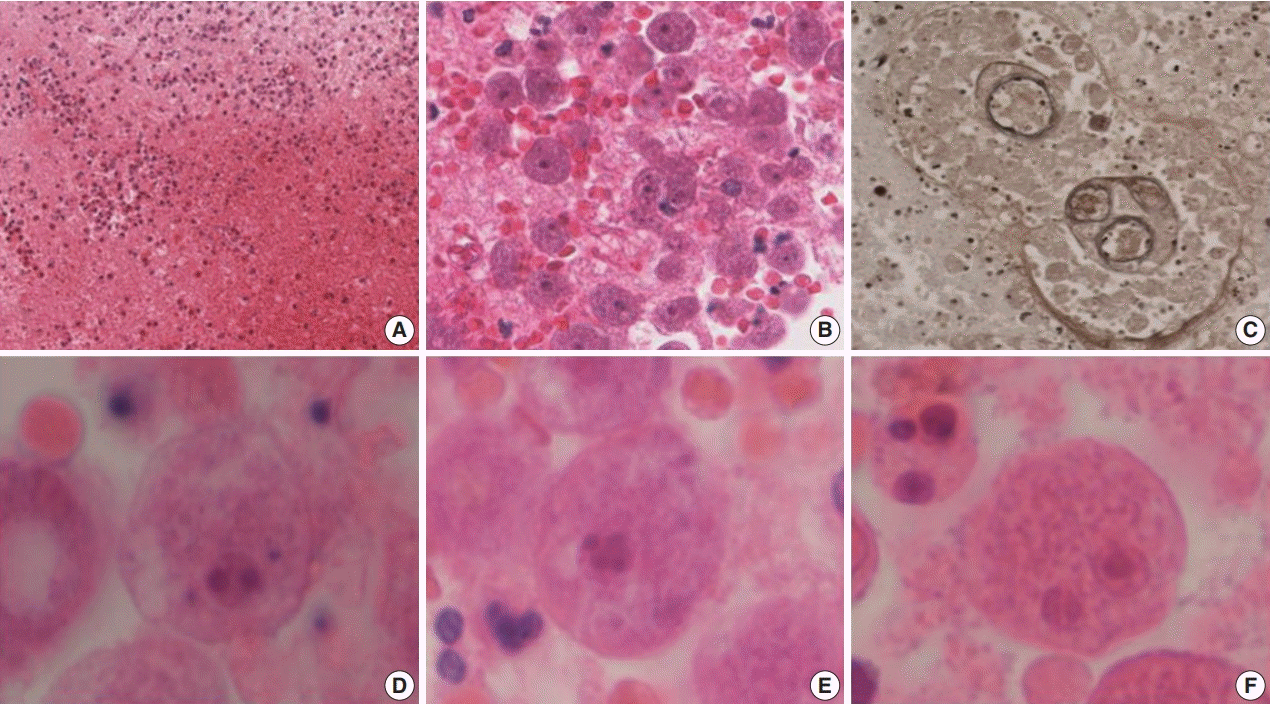
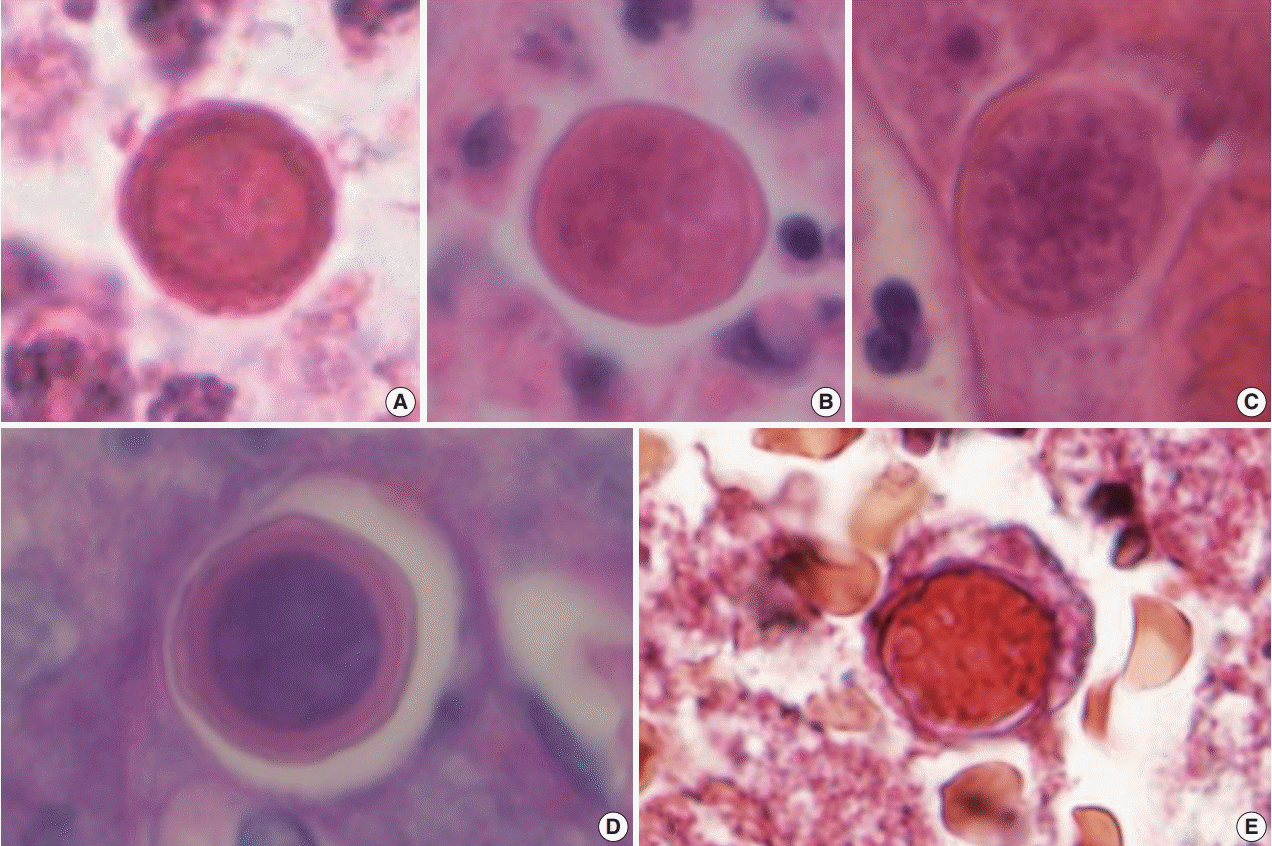
Therefore, an open biopsy of the brain was performed immediately. During the operation, the frozen sections with squash preparation showed acute inflammation with hemorrhagic necrosis, and no definitive diagnosis could be made. After the operation, the permanent sections showed diffuse or perivascular infiltration of amoebic trophozoites in the background of hemorrhage and necrosis (Fig. 2A–C). The trophozites were ovoid or round and 20–25 µm in size. They each had 1–2 nuclei containing 1–4 nucleoli (Fig. 2D–F). Additionally, some scattered cysts having inner rigid and outer wavy double-layered walls were observed (Fig. 3A–E). These findings were consistent with the diagnosis of B. mandrillaris amoebic encephalitis. Even though the patient was treated with the combination of pentamidine and paromomycin, he died on the 10th day after being admitted to the hospital.
This study was approved by the Institutional Review Board of Keimyung University Dongsan Medical Center, with waiver of informed consent (IRB No. 2019-02-023).
Go to : 
Balamuthia mandrillaris, Acanthamoeba species, and Naegleria fowleri are known to be free-living amoebae that can cause amoebic encephalitis. B. mandrillaris and Acanthamoeba species mainly cause granulomatous amoebic encephalitis, which shows subacute to chronic progression, while N. fowleri causes primary amoebic meningoencephalitis, which shows a highly acute progression [1]. Unfortunately, all of these amoebae do not have characteristic clinical features; thus, early diagnosis is very difficult for clinicians and the condition is associated with a high mortality rate of over 90% [5].
B. mandrillaris amoebic encephalitis is also a fatal condition, and the mortality rate is known to be 98% [3]. It affects not only immunocompromised but also immunocompetent individuals, especially the young and the old [2]. Despite its high mortality rate, the pathophysiology and pathogenesis of B. mandrillaris amoebic encephalitis are not clearly understood. However, it is assumed that the main routes of the infections are through the nasal mucosa or hematogenous spread through the respiratory and cutaneous pathways in a manner similar to other amoebic species. The CSF study did not reveal any organism, but showed a normal or slightly low glucose level and an elevated protein level, which are similar to findings with the presence of Acanthamoeba species [6].
Like Acanthamoeba species, B. mandrillaris also have two life cycle stages: trophozoite and cyst. Histopathologically, trophozoites have a round to ovoid shape and range from 12 to 60 µm in size. They usually only have a single nucleus, but, occasionally, more than one nucleus can be noticed. Each nucleus contains one to three nucleoli. When they become cysts, they have a triplelayered wall, but, under a light microscope, they are observed to have a double-layered wall: a round and rigid inner wall and wavy outer wall, ranging from 15–30 µm in size [7,8]. The cysts and trophozoites are prominent in the vascular or perivascular area, in the background of acute to granulomatous inflammation with angiitis, hemorrhage, and necrosis.
In contrast, Acanthamoeba species have vegetative trophozoites with cysts in the tissue. Trophozoites are 15–50 µm in size and contain one vesicular nucleus with one large, centrally placed nucleolus. Cysts range from 15–25 µm in size and have a doublelayered wall. The outer wall is wrinkled with folds and ripples, while the inner wall varies in shape. On the other hand, N. fowleri have only trophozoites without cysts in the brain tissue. Trophozoites are 10–25 µm in size and have one nucleus with one prominent nucleolus in the middle, and are usually observed around vessels, in the background of acute inflammation associated with hemorrhage and necrosis [8].
For the diagnosis of amoebic encephalitis, it is essential to identify organisms in the tissue or CSF [7,9]. Differential diagnosis of the three amoebae is possible using each of their characteristic histopathologic findings. In the present case, numerous trophozoites and scattered cysts were found in the vessel wall and perivascular area and were seen in association with acute inflammation and diffuse hemorrhagic necrosis. The trophozoites were in round to ovoid shape, having 1–2 nuclei, and each of them contained 1–4 nucleoli. The cysts were round to ovoid shape, had a double-layered wall, and measured 15–20 µm in size. While the inner layer was round and rigid, the outer layer was wavy, which is consistent with the features of B. mandrillaris. The characteristic histopathologic features of the three amoebae are listed in Table 1.
Differential diagnosis of amoebic encephalitis by histopathologic features
In the CSF study, the glucose level was in the normal range of 60 mg/dL and the protein level was elevated at 331.8 mg/dL, which is consistent with any of the amoebic encephalitis types. Unfortunately, a culture or pathologic smear of CSF was not performed. The CRP level in the blood was increased to 7.89 mg/ dL, and the white blood cell count was normal. Additionally, an immunofluorescence study or polymerase chain reaction can be used to identify the specific organism. However, in the present case, using only the amoeba-specific histopathologic features was sufficient to identify B. mandrillaris, and this emphasizes the importance of examining the histopathological features of the amoebae.
Go to : 
Notes
Author contributions
Conceptualization: SPK.
Data curation: SJK.
Formal analysis: HRJ, MC.
Investigation: SJK, HWL.
Project administration: SPK.
Supervision: SPK.
Validation: HWL, HRJ, MC.
Writing—original draft: SJK, SPK.
Writing—review & editing: SJK, HWL, HRJ, MC, SPK.
Go to : 
REFERENCES
1. Martinez AJ. Infection of the central nervous system due to Acanthamoeba. Rev Infect Dis. 1991; 13 Suppl 5:S399–402.

2. Diaz JH. The public health threat from Balamuthia mandrillaris in the southern United States. J La State Med Soc. 2011; 163:197–204.
3. Matin A, Siddiqui R, Jayasekera S, Khan NA. Increasing importance of Balamuthia mandrillaris. Clin Microbiol Rev. 2008; 21:435–48.
4. Itoh K, Yagita K, Nozaki T, et al. An autopsy case of Balamuthia mandrillaris amoebic encephalitis, a rare emerging infectious disease, with a brief review of the cases reported in Japan. Neuropathology. 2015; 35:64–9.
5. Diaz JH. Behavioral and recreational risk factors for free-living amebic infections. J Travel Med. 2011; 18:130–7.

6. Kiderlen AF, Laube U. Balamuthia mandrillaris, an opportunistic agent of granulomatous amebic encephalitis, infects the brain via the olfactory nerve pathway. Parasitol Res. 2004; 94:49–52.

7. Visvesvara GS, Schuster FL, Martinez AJ. Balamuthia mandrillaris, N. G., N. Sp., agent of amebic meningoencephalitis in humans and other animals. J Eukaryot Microbiol. 1993; 40:504–14.
8. Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007; 50:1–26.
9. Reddy R, Vijayasaradhi M, Uppin MS, Challa S, Jabeen A, Borghain R. Acanthamoeba meningoencephalitis in an immunocompetent patient: an autopsy case report. Neuropathology. 2011; 31:183–7.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download