1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.

2. Azodi M, Langer A, Jenison EL. Primary fallopian tube carcinoma with isolated torsion of involved tube. Eur J Gynaecol Oncol. 2000; 21:364–7.
3. Singhal P, Odunsi K, Rodabaugh K, Driscoll D, Lele S. Primary fallopian tube carcinoma: a retrospective clinicopathologic study. Eur J Gynaecol Oncol. 2006; 27:16–8.
4. Kalampokas E, Sofoudis C, Boutas I, Kalampokas T, Tourountous I. Primary fallopian tube carcinoma: a case report and mini-review of the literature. Eur J Gynaecol Oncol. 2014; 35:595–6.
5. Riska A, Leminen A. Updating on primary fallopian tube carcinoma. Acta Obstet Gynecol Scand. 2007; 86:1419–26.

6. Callahan MJ, Crum CP, Medeiros F, et al. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol. 2007; 25:3985–90.

7. Kalampokas E, Kalampokas T, Tourountous I. Primary fallopian tube carcinoma. Eur J Obstet Gynecol Reprod Biol. 2013; 169:155–61.

8. Kessler M, Fotopoulou C, Meyer T. The molecular fingerprint of high grade serous ovarian cancer reflects its fallopian tube origin. Int J Mol Sci. 2013; 14:6571–96.

9. Reade CJ, McVey RM, Tone AA, et al. The fallopian tube as the origin of high grade serous ovarian cancer: review of a paradigm shift. J Obstet Gynaecol Can. 2014; 36:133–40.

10. Kim J, Coffey DM, Creighton CJ, Yu Z, Hawkins SM, Matzuk MM. High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc Natl Acad Sci U S A. 2012; 109:3921–6.

11. Przybycin CG, Kurman RJ, Ronnett BM, Shih IM, Vang R. Are all pelvic (nonuterine) serous carcinomas of tubal origin? Am J Surg Pathol. 2010; 34:1407–16.

12. Riska A, Leminen A, Pukkala E. Sociodemographic determinants of incidence of primary fallopian tube carcinoma, Finland 1953-97. Int J Cancer. 2003; 104:643–5.

13. Potapov S, Sidorenko R, Galata D, Stratiy N, Gargin V. Peculiarities of catenin activity in the embryonal testicular carcinoma. Georgian Med News. 2016; (261):68–73.
14. Romaniuk A, Lyndin M, Smiyanov V, Sikora V, Rieznik A, Kuzenko Y, et al. Primary multiple tumor with affection of the thyroid gland, uterus, urinary bladder, mammary gland and other organs. Pathol Res Pract. 2017; 213:574–9.

15. Oliveira C, Duarte H, Bartosch C, Fernandes D. Small fallopian tube carcinoma with extensive upper abdominal dissemination: a case report. J Med Case Rep. 2013; 7:252.

16. Pectasides D, Pectasides E, Papaxoinis G, et al. Primary fallopian tube carcinoma: results of a retrospective analysis of 64 patients. Gynecol Oncol. 2009; 115:97–101.

17. Wang Y, Li L, Wang Y, Tang SN, Zheng W. Fallopian tube secretory cell expansion: a sensitive biomarker for ovarian serous carcinogenesis. Am J Transl Res. 2015; 7:2082–90.
18. Wang Y, Wang Y, Li D, et al. IMP3 signatures of fallopian tube: a risk for pelvic serous cancers. J Hematol Oncol. 2014; 7:49.

19. Farooq S, Tasleem R, Nazir N, Reshi R, Hassan Z. Histopathological pattern of ovarian neoplasms and estrogen and progesterone receptor expression in primary epithelial tumours and their histopathological correlation. Int J Curr Res Rev. 2013; 5:70–7.
20. Rose PG, Piver MS, Tsukada Y. Fallopian tube cancer. The Roswell Park experience. Cancer. 1990; 66:2661–7.

21. Rosen AC, Reiner A, Klein M, et al. Prognostic factors in primary fallopian tube carcinoma. Austrian Cooperative Study Group for Fallopian Tube Carcinoma. Gynecol Oncol. 1994; 53:307–13.
22. Thomas C, Gustafsson JÅ. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer. 2011; 11:597–608.

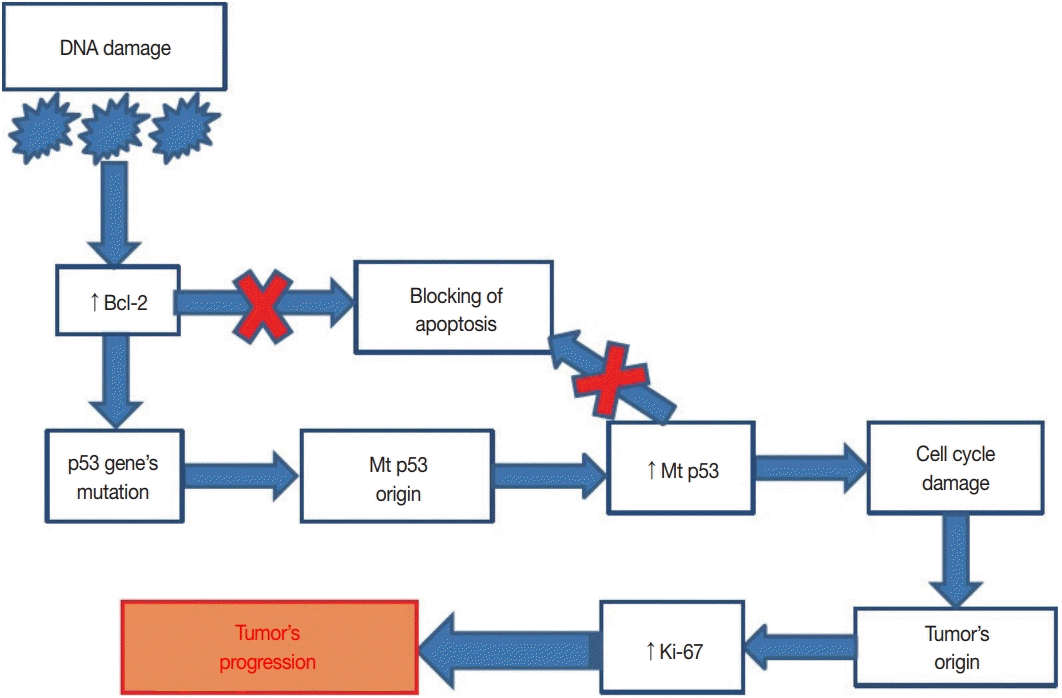
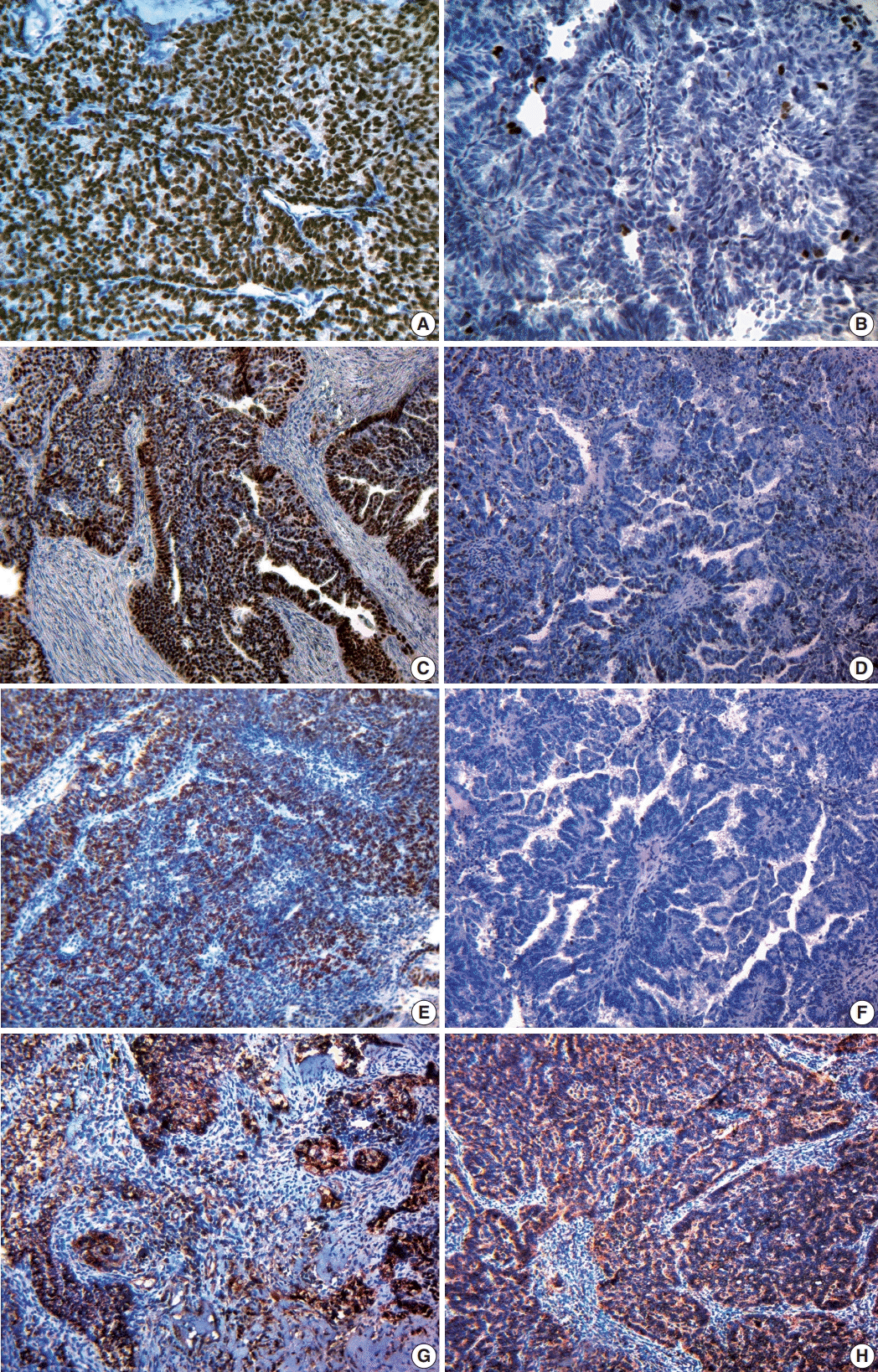
23. Lacy MQ, Hartmann LC, Keeney GL, et al. c-erbB-2 and p53 expression in fallopian tube carcinoma. Cancer. 1995; 75:2891–6.

24. Alvarado-Cabrero I, Kiyokawa T, Piña P, Valencia-Cedillo R, Santiago-Payán H, Stolnicu S. HER2/neu, p53, MIB1 and PAX8 immunoexpression in primary serous fallopian tube carcinomas. Rev Esp Patol. 2016; 49:219–25.

25. Chung TK, Cheung TH, To KF, Wong YF. Overexpression of p53 and HER-2/neu and c-myc in primary fallopian tube carcinoma. Gynecol Obstet Invest. 2000; 49:47–51.
26. Dawson SJ, Makretsov N, Blows FM, et al. BCL2 in breast cancer: a favourable prognostic marker across molecular subtypes and independent of adjuvant therapy received. Br J Cancer. 2010; 103:668–75.

27. Romaniuk A, Lyndin M. Immune microenvironment as a factor of breast cancer progression. Diagn Pathol. 2015; 10:79.

28. Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005; 69 Suppl 3:4–10.

29. Lichtenberger BM, Tan PK, Niederleithner H, Ferrara N, Petzelbauer P, Sibilia M. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell. 2010; 140:268–79.

30. Lytvynenko M, Bocharova T, Zhelezniakova N, Narbutova T, Gargin V. Cervical transformation in alcohol abuse patients. Georgian Med News. 2017; (271):12–7.
31. Lytvynenko M, Shkolnikov V, Bocharova T, Sychova L, Gargin V. Peculiarities of proliferative activity of cervical squamous cancer in HIV infection. Georgian Med News. 2017; (270):10–5.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download