It is widely known that there are two morphologic multistep colorectal carcinogenesis pathways: the conventional pathway and the serrated pathway [
1,
2]. In the conventional pathway, colorectal carcinomas (CRCs) develop through premalignant lesions, including tubular, tubulovillous, and villous adenomas, accounting for approximately 60%–80% of the CRCs, whereas in the serrated pathway, about 15%–35% of the CRCs progress from serrated precursor lesions [
1,
3,
4]. According to the latest (4th) edition of the World Health Organization (WHO) classification of tumors of the digestive system, serrated colorectal lesions are classified into three categories: hyperplastic polyps (HPs), sessile serrated adenomas/polyps (SSA/Ps), and traditional serrated adenomas (TSAs) [
5]. Among the three subtypes of serrated lesions, SSA/Ps and TSAs are recognized as premalignant lesions, whereas HPs are not regarded as direct precursors of carcinoma but rather as potential precursors of SSA/Ps or TSAs [
5,
6].
It has been suggested that risk factors to predict the progression of SSA/Ps are morphologic dysplasia, size of the polyp, and the number of serrated polyps [
6,
10]. Based on these potential risk factors, endoscopic surveillance guidelines have generally recommended shorter surveillance interval for SSA/Ps with morphologic dysplasia, large polyp size (≥ 10 mm), or multiple SSA/Ps or TSAs (3 or more) [
6]. However, although SSA/Ps without dysplasia are more prevalent than SSA/Ps with dysplasia, there has been a lack of reliable grading or risk-stratifying system for non-dysplastic SSA/Ps. Therefore, identifying clinicopathologic factors correlated with underlying molecular alterations that indicate high-risk lesions would be important for establishing a precise risk-assessment system for non-dysplastic SSA/Ps.
DISCUSSION
Until recently, number (many), size (large), anatomic site (right-sided colon), and morphologic dysplasia of SSA/Ps have been regarded as the potential risk factors for the progression of SSA/Ps into carcinomas [
6,
10]. However, among these potential risk factors, only morphologic dysplasia is based on strong molecular and pathologic evidence, such as high incidences of MLH1 deficiency, CIMP-high, and accompanying carcinomatous component in SSA/Ps with dysplasia [
6,
20,
21]. The other potential risk factors for malignant change of SSA/Ps, including number, size, and location of SSA/Ps, have been suggested by experts, based mainly on less-than-robust, inconclusive clinicopathologic data [
6,
10]. Moreover, the ‘cutoff’ values for the number and size of SSA/Ps for the prediction of high-risk lesions have not been clearly established. Therefore, there is a strong need to precisely identify clinicopathologic risk factors for carcinomatous change in SSA/Ps, especially in SSA/Ps without dysplasia. As no official grading or risk-stratifying system exists for non-dysplastic SSA/Ps, investigating the clinicopathologic factors significantly associated with a high-risk subgroup of non-dysplastic SSA/Ps would be a worthwhile study for clinical and pathologic practices.
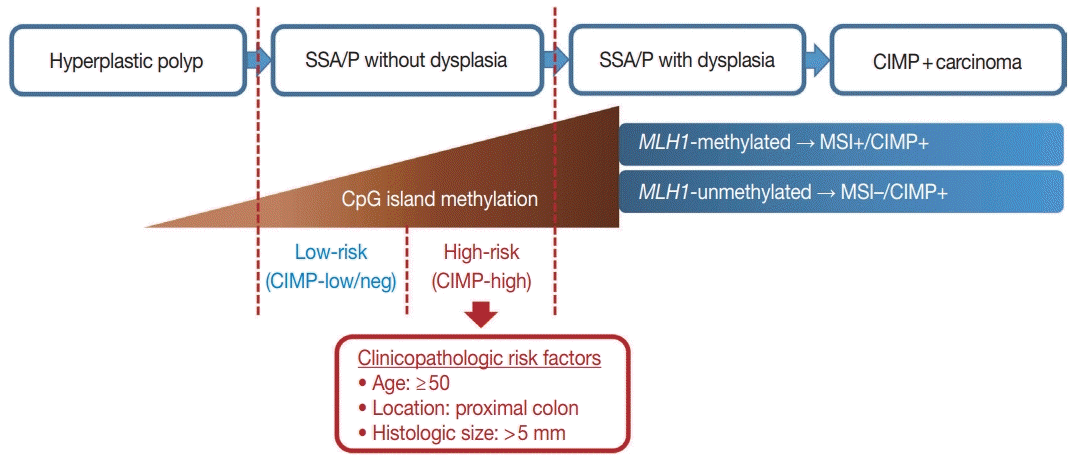
To define a high-risk subgroup of non-dysplastic SSA/Ps, we used molecular criteria (CIMP-high and/or
MLH1 promoter methylation), because the accumulation of CpG island methylation is known as a molecular hallmark of progression of SSA/Ps (
Figs. 2B,
5) [
11,
12]. Based on this aspect, both CIMP-high and
MLH1 methylation in SSA/Ps indicate lesions that are epigenetically high-potential to develop into carcinoma. CIMP-high is known to be tightly associated with
MLH1 methylation in CRCs, and
MLH1 has been used as one of the essential markers for determination of CIMP-high status in CRCs [
15,
22]. MLH1 deficiency via the methylation of its gene promoter can induce high microsatellite instability (MSI-high) and subsequent genome-wide hypermutated status in the tumor cells of premalignant lesions such as SSA/Ps [
8], and the hypermutated premalignant lesions can rapidly progress into carcinomas [
6,
10,
21].
Fig. 5.
A graphical summary of this study. Age, location, and size are potential predictive factors for a high-risk subgroup of non-dysplastic sessile serrated adenomas/polyps (SSA/Ps). CIMP, CpG island methylator phenotype; MSI, microsatellite instability.

In our present study, a total of 132 non-dysplastic SSA/Ps were classified into 34 high-risk and 98 low-risk lesions based on the CpG island methylation criteria (CIMP-high and/or
MLH1 methylation). We performed statistical analyses using various clinicopathologic factors and found three major factors for potential use as predictive factors for high-risk lesions of non-dysplastic SSA/Ps: patient age, lesion location, and histologically measured lesion size (
Fig. 5).
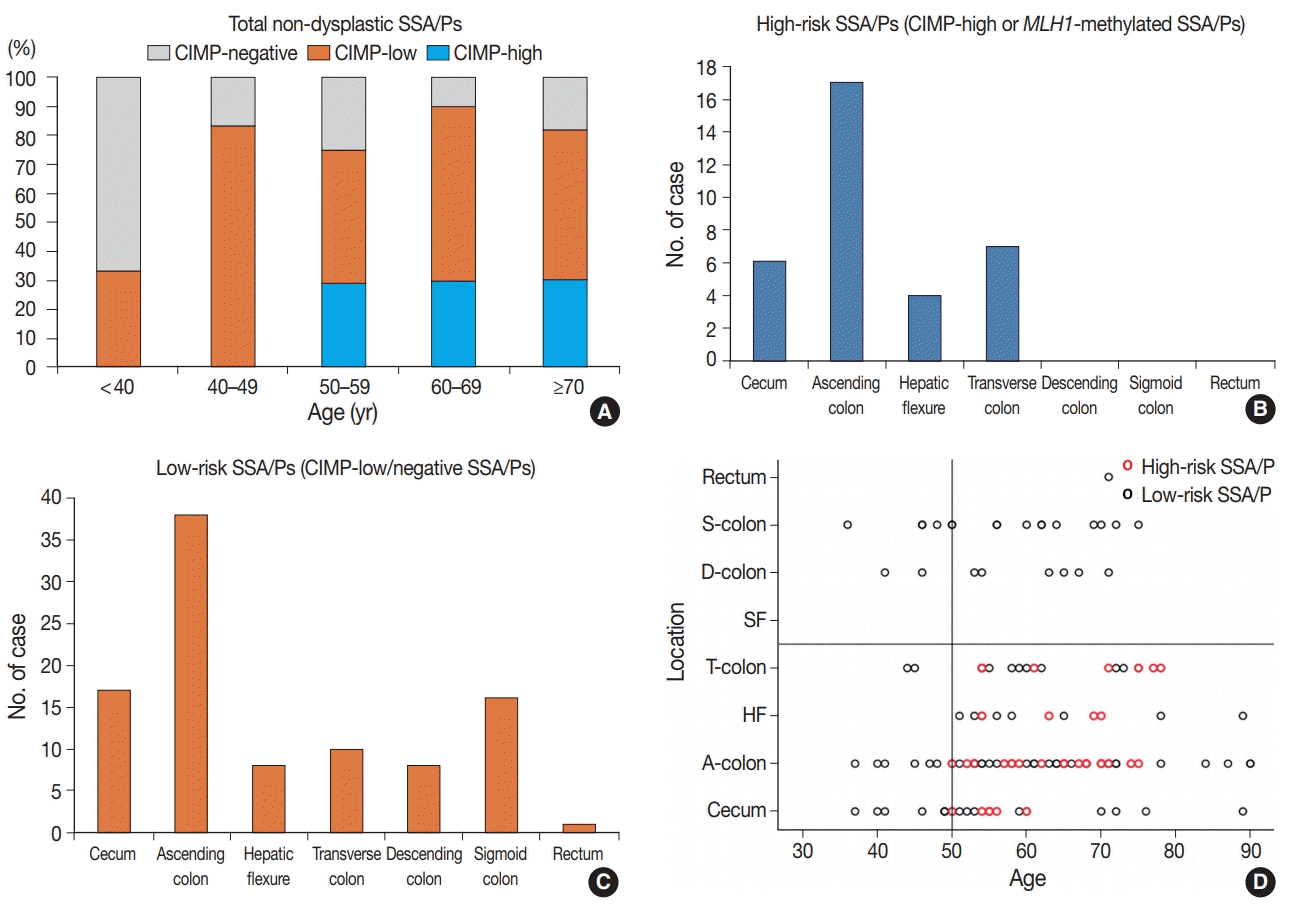
First, age is a significant factor for molecularly defined high-risk SSA/Ps. According to our results, the high-risk SSA/Ps were found exclusively in patients aged 50 years or more (
Table 1,
Fig. 3A,
D). This finding suggests that most of the SSA/Ps found in individuals under the age of 50 may be molecularly benign lesions, with a low risk of progression into malignancy. In fact, the present as well as previous studies suggest that age is important for understanding the characteristics of CpG island methylation in SSA/Ps. Liu et al. [
23] recently published data highlighting the importance of age as a risk factor for CIMP-high and malignant progression in SSA/Ps [
24]. According to these studies, SSA/Ps in young patients rarely demonstrate CIMP-positive (CIMP-high) status, indicating a limited risk of malignant change in SSA/Ps of young patients [
23,
24]. These results are very similar to our data. If consistent data are accumulated in future studies as well, it would strengthen the case for using age as an important risk-predictive factor in surveillance and diagnostic guidelines for SSA/Ps.
Our study also revealed that the lesion location could be a significant factor for the high-risk subgroup of non-dysplastic SSA/Ps. All 34 high-risk SSA/Ps were located exclusively in the proximal colon, including cecum, ascending colon, hepatic flexure, and transverse colon (
Table 1,
Fig. 3B,
D). This finding is not surprising, as previous studies have also suggested that the proximal colonic location is important for malignancy risk of SSA/Ps [
6,
10]. The recent study by Liu et al. [
23] found that CIMP-high was significantly correlated with proximal location in SSA/Ps, although a small number of distal-located SSA/Ps were also determined to be CIMP-high lesions. Through our results, the importance of lesion location in the prediction of malignant potential of SSA/Ps has been further confirmed.
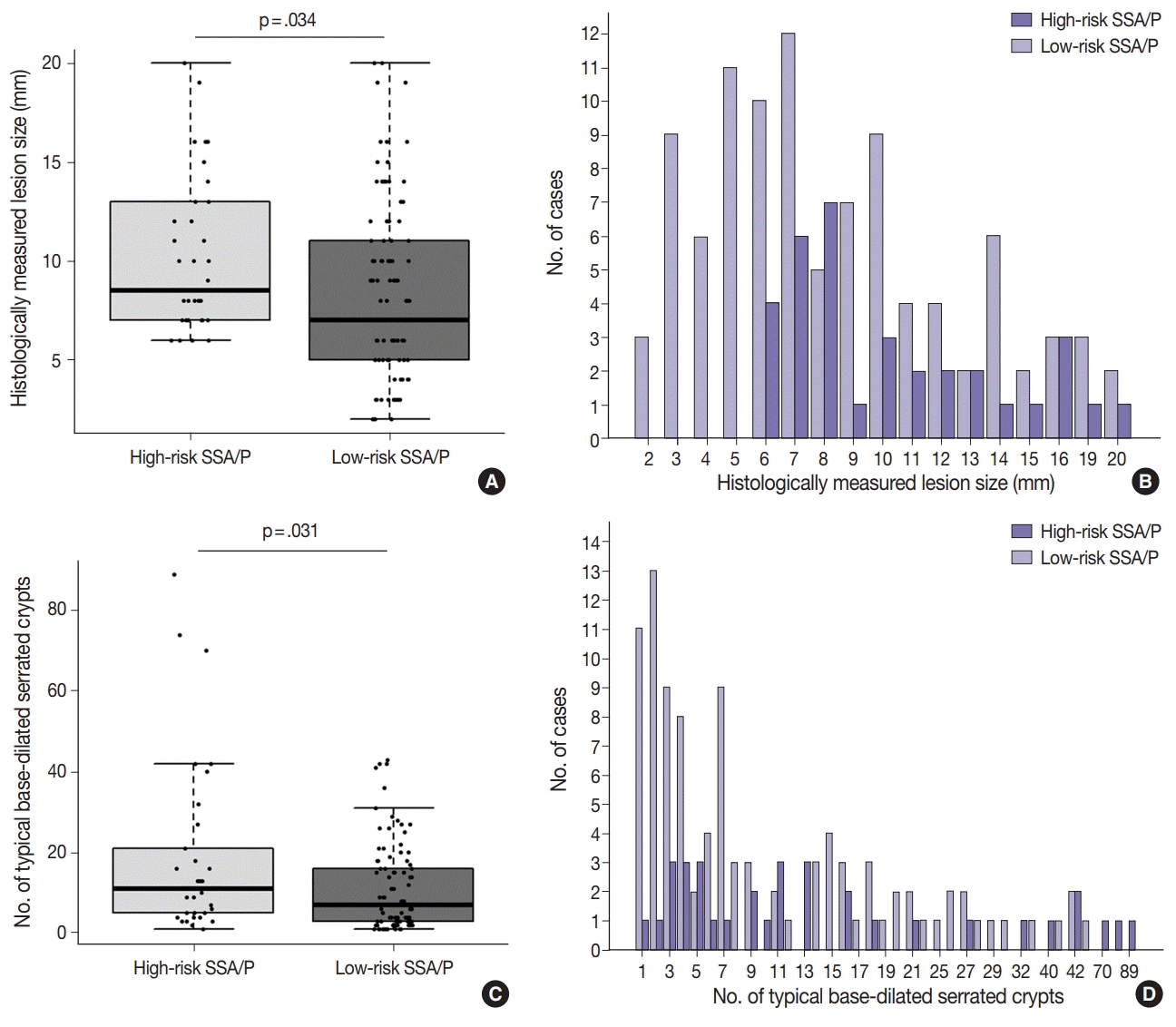
In addition to patient age and lesion location, histologically measured lesion size was also a significant factor for high-risk SSA/Ps without dysplasia. All 34 high-risk SSA/Ps measured at least 6 mm in size, and the differences of histologically measured sizes between high-risk and low-risk SSA/Ps were statistically significant (
Table 1,
Fig. 4A,
B). Notably, lesion size measured by endoscopy was not a statistically significant factor for the discrimination of high-risk lesions from low-risk SSA/Ps (
Table 1,
Supplementary Fig. S6). Although, similar to histologically measured lesion size, the endoscopically measured lesion size of all 34 high-risk SSA/Ps was at least 5 mm, the majority of low-risk SSA/Ps (93%) also demonstrated a size of 5 mm or more, when measured by endoscopy (
Table 1), which likely led to the lack of statistical significance. These findings collectively indicate that histologically measured lesion size may be more valuable and crucial for the risk evaluation of SSA/Ps than endoscopically measured size. The superior value of lesion size measured by histomorphologic methods in the prediction of high-risk SSA/Ps should be validated by multiple independent studies in the future.
As mentioned earlier, although the multiplicity of serrated lesions has been regarded as a potential risk factor for malignant progression of SSA/Ps [
6], the increased number of synchronous serrated lesions was not a significant factor for high-risk non-dysplastic SSA/Ps in our data (
Table 1,
Supplementary Fig. S3). In fact, because our study excluded cases satisfying diagnostic criteria for serrated polyposis, the expected high-risk lesions in serrated polyposis might be substantially removed from our study samples. As SSA/Ps in serrated polyposis might be clinicopathologically and molecularly different from sporadic SSA/Ps [
5,
25,
26], we decided to focus on sporadically arising SSA/Ps in this study. Therefore, the value of multiplicity of serrated lesions in the prediction of high-risk SSA/Ps should not be underestimated based on the results of our study alone. Nevertheless, our data tend to suggest that multiplicity of serrated lesions may be less valuable than age, location, or size, for prediction of high-risk lesions among sporadic SSA/Ps.
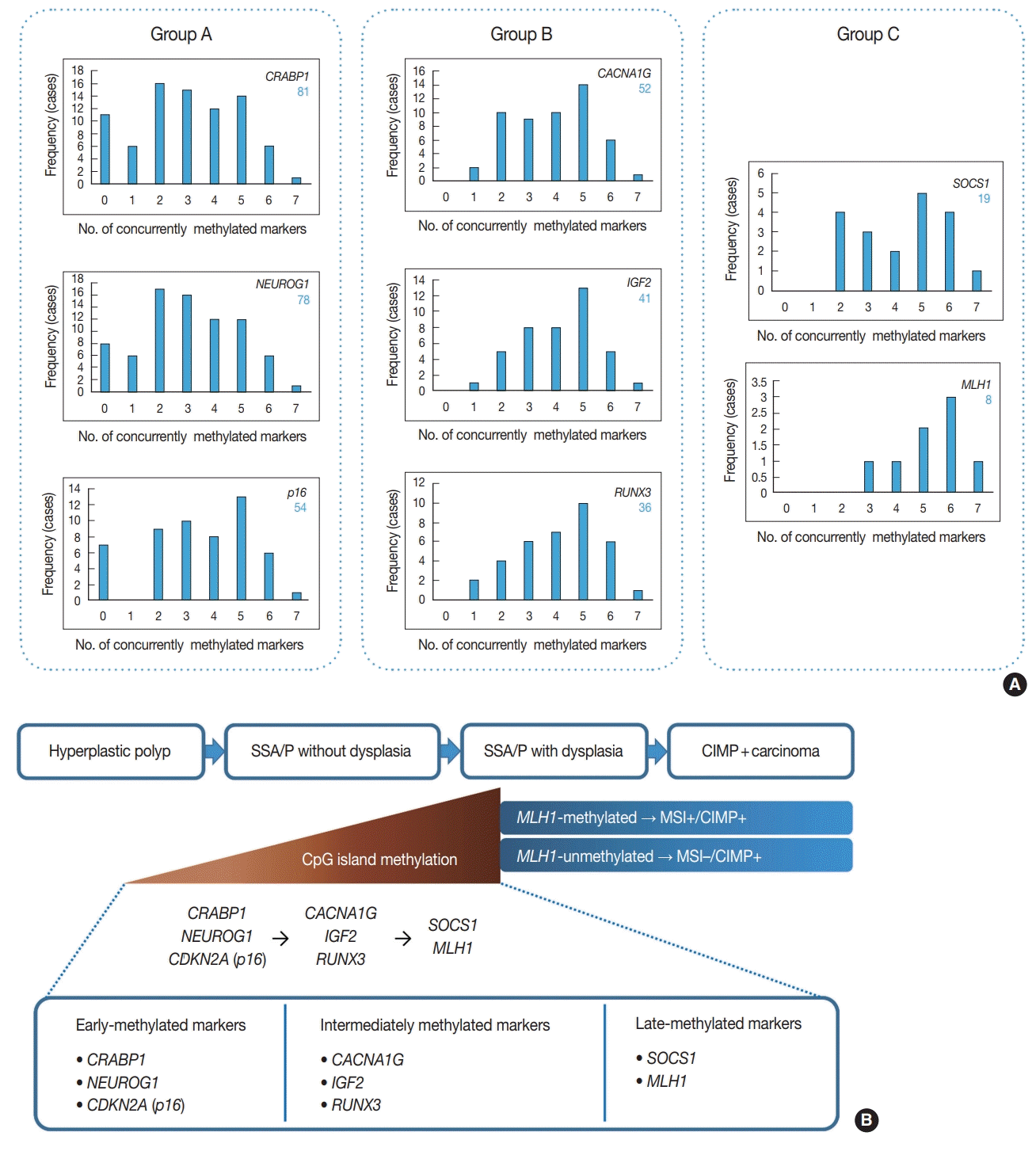
In addition to the identification of clinicopathologic factors associated with the high-risk subgroup of non-dysplastic SSA/Ps, the elucidation of developmental pattern of CpG island methylation in SSA/Ps is another important finding from our study. Based on concurrently methylated marker-dependent and age-dependent frequencies of methylation of each CIMP marker in 132 non-dysplastic SSA/Ps (
Fig. 2A,
Supplementary Fig. S1), we suggested a sequential model of methylation of CIMP markers in serrated neoplasia pathway (
Fig. 2B). Early-methylated markers such as
CRABP1 and
NEUROG1 could be methylated in SSA/Ps of young patients, whereas late-methylated markers such as
SOCS1 and
MLH1 could get methylated only in SSA/Ps of patients over the age of 50 (
Supplementary Fig. S1). These results indicate that the accumulation of CpG island methylation during the progression of SSA/Ps is not a stochastic process, but rather an age-dependent, directed process. Our finding is valuable in understanding the characteristics of epigenetic alterations in colorectal carcinogenesis, because currently, the detailed pattern of CIMP development in serrated neoplasia pathway is poorly understood.
Promoter CpG island methylation in
MLH1 gene is a critical step for the development of MSI-high/CIMP-high CRCs from SSA/Ps [
8]. When
MLH1 is methylated and downregulated in an SSA/P without dysplasia, MSI-high and hypermutated phenotype can be induced in the genome of the SSA/P, and the non-dysplastic SSA/P can rapidly progress into dysplasia and carcinoma [
6,
10,
21]. Based on our results, it can be inferred that the following two conditions should be satisfied for the
MLH1 gene to be methylated in non-dysplastic SSA/Ps: (1) other CIMP markers sufficiently methylated in an SSA/P (generally three or more methylated markers) (
Fig. 2A), and (2) old age of the patient (generally ≥ 50 years old) (
Supplementary Fig. S1). We also found that both complete loss of MLH1 expression and morphologic dysplasia may occur in SSA/Ps, when
MLH1 promoter CpG islands are sufficiently methylated (
Supplementary Table S1).
MLH1 methylations in non-dysplastic SSA/Ps are mainly subrepressive alterations, because all
MLH1-methylated non-dysplastic SSA/Ps demonstrated relatively low PMR values and did not show a complete loss of MLH1 expression (
Supplementary Table S1,
Supplementary Fig. S2). Thus, MLH1 IHC may not be useful for the screening of high-risk lesions among non-dysplastic SSA/Ps.
In conclusion, CpG island methylation may be an age-dependent stepwise process in the colorectal serrated neoplasia pathway. Both CIMP-high and MLH1 methylation are late-step alterations during the progression of SSA/Ps and are unlikely to occur in SSA/Ps of young patients. Proximal colon-located, > 5 mm-sized SSA/Ps found in individuals aged ≥ 50 should be considered as potential high-risk lesions, regardless of morphologic dysplasia or lesion multiplicity (
Fig. 5).




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download