CASE REPORT
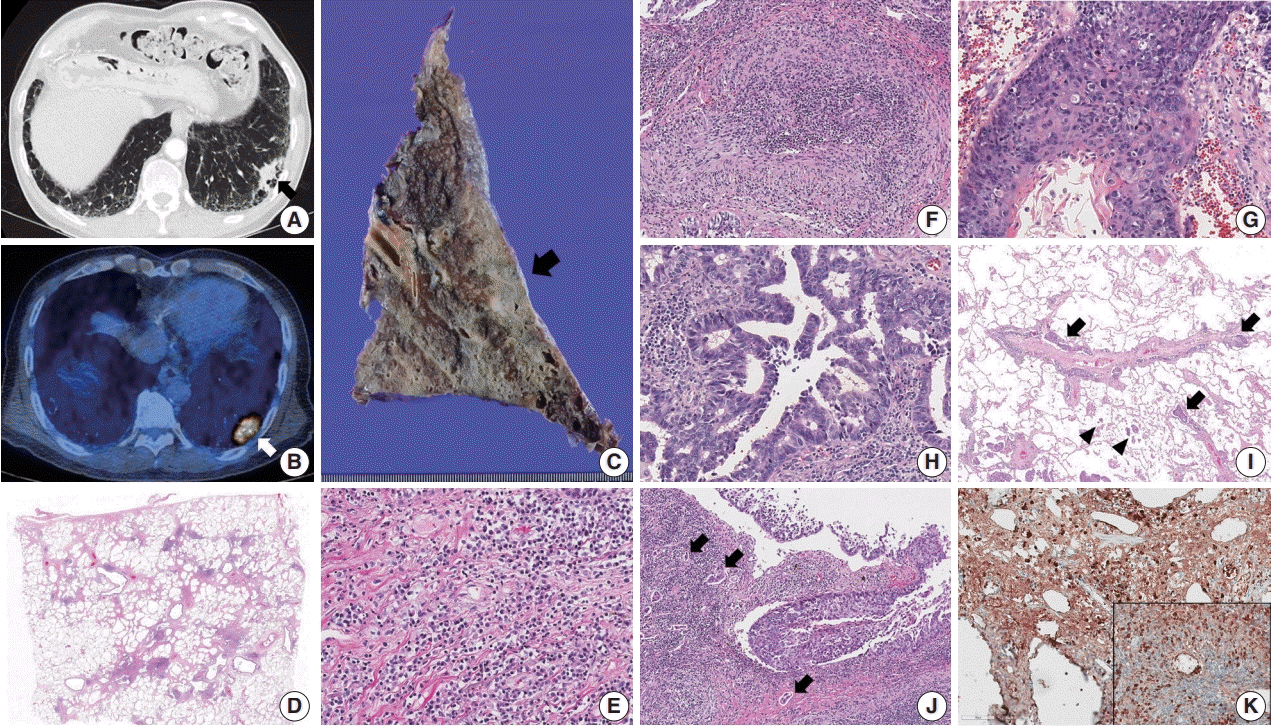 | Fig. 1.(A, B) Chest computed tomography shows a consolidative nodule (arrow) in a background of subpleural reticulation and honeycomb fibrosis at both lung bases. Positron emission tomography reveals 18F-fluorodeoxyglucose uptake by the nodule. (C) The cut section of the lung showed an ill-defined and gray-tan colored mass (arrow). The background lung was emphysematous and fibrotic. (D–F) Histologic examination shows irregular interstitial fibrosis with patchy lymphoid aggregation, predominant lymphoplasmacytic infiltration, and occasional obliterative phlebitis. (G) The squamous cell carcinoma component shows keratinization and multifocal dyskeratosis. (H) The adenocarcinoma component was mainly composed of a moderately differentiated acinar pattern. (I) Diffuse spread through air space (arrowheads) and multifocal lymphangitic spread (arrow) of tumor cells are frequently found at the periphery of the mass. (J) Dense fibrosis and lymphoplasmacytic infiltration are found in the peritumoral area. Multifocal endolymphatic tumor emboli (arrows) are also noted. (K) Both IgG4 and IgG (inset) immunohistochemical stains show diffuse positivity in the infiltrating plasma cells. The IgG4/IgG ratio was over 40%. |
DISCUSSION
Table 1.
| Reference | Sex | Age (yr) | Location | Type of tumor | Pattern of ADC | Radiologic finding | Pattern of IgG4-RLD | TNM stage | Other manifestations | Serum IgG4 (mg/dL) |
|---|---|---|---|---|---|---|---|---|---|---|
| Present case | M | 66 | LLL | ASC | Acinar and focal micropapillary | Subpleural nodule in a background of reticular and honeycomb fibrosis | Interstitial | pT2aN2M0 | IHD | 232 |
| Zen et al. [4] | M | NA | RLL | ADC | Mixed, including acinar | Nodular lesion within the reticular shadow | Interstitial | pT1N2M0 | No | NA |
| Inoue et al. [5] | M | 78 | RUL | ADC | Lepidic | Ground-glass opacity with central collapse and pleural indentation | Nodular | pT1bN0M0 | Pancreas | 983 |
| Tashiro et al. [6] | M | 72 | RML | ADC | Lepidic | Spiculated nodule with pleural indentation | Nodular | pT1bN0M0 | No | 346 |
IgG4-RLD, IgG4-related lung disease; ADC, adenocarcinoma; M, male; LLL, left lower lobe; ASC, adenosquamous carcinoma; Interstitial, interstitial lung disease type; IHD, intrahepatic bile duct; RLL, right lower lobe; NA, not available; RUL, right upper lobe; Nodular, solid nodular type; RML, right middle lobe.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download