DISCUSSION
Direct or indirect roles of gut microbiota in the pathogenesis of a variety of human diseases have been recently proposed. The demonstration of the close association between
F. nucleatum and CRC has prompted exploration of the pathogenetic, prognostic, and predictive roles of
F. nucleatum in CRC. However, there are still limited data regarding the prognostic and predictive values of
F. nucleatum in CRC. Several studies using clinical samples have indicated that intratumoral
F. nucleatum is potentially associated with poor prognosis in CRC patients [
3,
11,
20]. Moreover, an experimental study suggested that
F. nucleatum might be able to induce resistance to chemotherapy by modulating autophagy in CRC cells [
4]. Based on the emerging prognostic significance and potential predictive value of
F. nucleatum in CRC, we decided to investigate the prognostic relevance of
F. nucleatum in CRCs treated with adjuvant chemotherapy. Most patients with stage III or high-risk stage II CRC are treated with adjuvant chemotherapy after curative surgery to prevent tumor recurrence. Thus, we collected a large series of stage III or high-risk stage II CRCs treated with oxaliplatin-based adjuvant chemotherapy. The survival differences in patient subgroups according to DNA amount of intratumoral
F. nucleatum measured by qPCR were statistically analyzed. We found that a high load of intratumoral
F. nucleatum was independently correlated with improved survival in patients with stage II/III non-sigmoid colon cancer treated with oxaliplatinbased adjuvant chemotherapy (
Table 2).
There is a discrepancy between our research and previous studies. Several previous studies revealed that
F. nucleatum–high CRC patients group tended to have shorter disease-specific survival than
F. nucleatum–low/negative CRC patients group [
3,
11,
20]. However, in the current study,
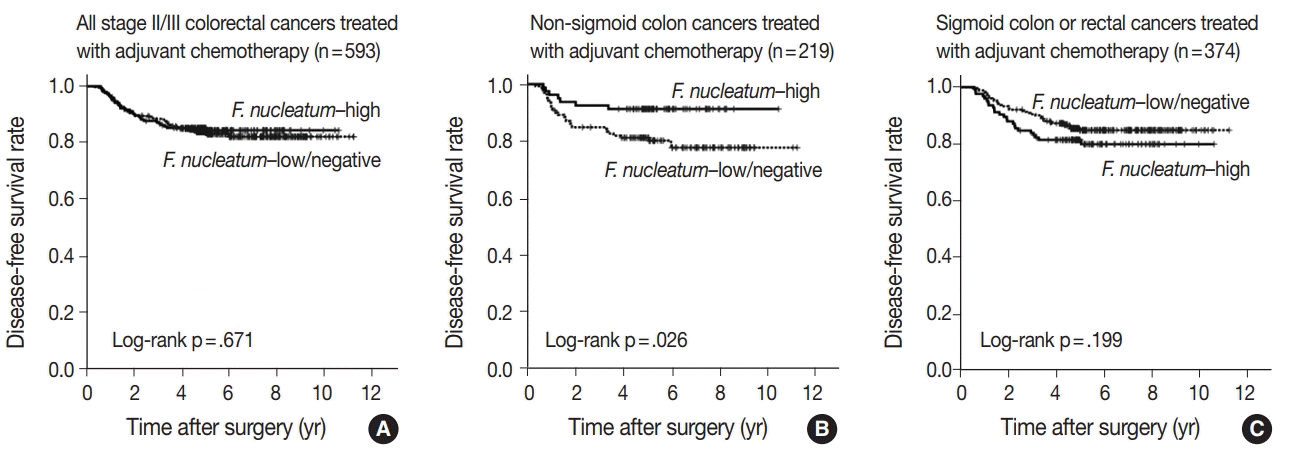
F. nucleatum had different prognostic impacts based on tumor location in CRCs treated with adjuvant chemotherapy. In detail, tumors with high levels of
F. nucleatum had better prognosis than those with low or negative levels of
F. nucleatum in non-sigmoid colon cancers, including cecum, ascending colon, transverse colon, and descending colon cancers (
Table 2,
Fig. 2B). On the other hand,
F. nucleatum–high CRCs showed a tendency toward worse prognosis compared to
F. nucleatum–low/negative CRCs in sigmoid colon and rectal cancers (
Fig. 2C). Since these contrasting prognostic implications of
F. nucleatum according to tumor location may counterbalance the overall prognostic effect of
F. nucleatum in CRCs, presently
F. nucleatum displayed no association with prognosis in a total of 593 stage II/III CRC patients treated with adjuvant chemotherapy (
Fig. 2A). The reason for the discrepancy between the current and prior findings may be the difference in the composition of the study populations. Yamaoka et al. [
20] described that
F. nucleatum was highly correlated with shorter disease specific survival especially in stage IV CRCs. In that study, in all stages of CRCs, disease-specific survival was decreased in CRCs featuring a high level of
F. nucleatum compared with that in CRCs with low levels of
F. nucleatum, although the survival differences according to
F. nucleatum level was decreased compared to that in the stage IV CRC subgroup [
20]. In addition, it cannot be excluded that there might be heterogeneities of detailed treatment approaches, such as adjuvant chemotherapy regimen, in the CRC cohorts of other studies. By contrast, our study samples were a well-selected and relatively-homogeneous cohort that contained only stage III or high-risk stage II CRCs treated with oxaliplatin-based adjuvant chemotherapy. Therefore, the prognostic implications of
F. nucleatum in CRC that is evident from our study could be meaningfully different from the results of other research groups.
In an experimental study,
F. nucleatum promoted resistance to chemotherapy in CRC cells [
4]. However, our results indicate that influences of
F. nucleatum on responses to chemotherapy might be diverse in the context of tumor location of CRCs. In sigmoid colon and rectal cancers, the expected chemoresistant effect of
F. nucleatum seems to have occurred because
F. nucleatum–high was linked with poor prognosis in sigmoid colon and rectal cancer patients treated with adjuvant chemotherapy, although statistical significance was not reached (
Fig. 2C). Nevertheless, in nonsigmoid colon cancers, a chemoresistant role of
F. nucleatum seems to be attenuated. Rather,
F. nucleatum might induce a chemoresponsive effect because
F. nucleatum–high was significantly associated with favorable prognosis in non-sigmoid colon cancers treated with adjuvant chemotherapy (
Table 2,
Fig. 2B). The underlying mechanism of the potential contrasting effects of
F. nucleatum on the chemotherapy response depending on location of CRC is unclear. However, the idea that different tumor locations can define different prognosis and treatment responses in CRC has been increasingly addressed. In fact, based on the accumulating clinical data, primary tumor location is regarded as a prognostic factor in metastatic CRCs [
21]. Stage IV CRCs primarily located in the right-sided colon are significantly associated with worse prognosis compared with left-sided stage IV CRCs. The different molecular, pathological, and clinical features between right-sided colon cancers and left-sided CRCs have been reported [
21,
22]. Therefore, the potential different impacts of
F. nucleatum on prognosis and treatment responses according to tumor location in CRCs are not surprising. To the best of our knowledge, this study is the first report to investigate the prognostic effect of
F. nucleatum according to tumor location in CRCs, especially in adjuvant chemotherapy-treated CRCs. Our study suggests that the prognostic effect of
F. nucleatum should be evaluated considering the location of the tumor.
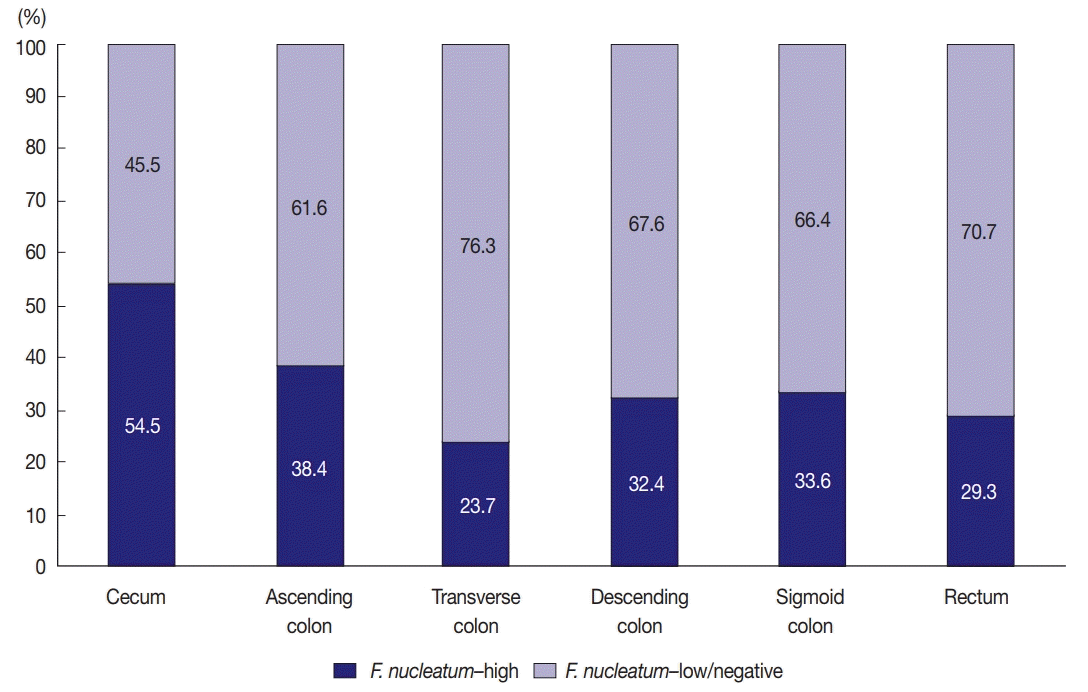
In this study, the proportion of
F. nucleatum–high CRCs differed in each tumor location bowel subsite. The proportion of
F. nucleatum–high tumors in all the CRCs was 34.4% (204 of 593). Cecal cancers displayed the highest proportion of
F. nucleatum-high tumors (54.5%), followed by ascending colon cancers (38.4%) (
Fig. 1). It was notable that over half of the cecal cancers were
F. nucleatum–high tumors. Our results are consistent with those of previous studies demonstrating the significant association of the proximal location of CRCs with a high level of intratumoral
F. nucleatum. According to the study by Mima et al. [
13], the proportion of
F. nucleatum–high CRCs increased along the distance from the anal verge, and cecal cancers showed the highest proportion of
F. nucleatum–high subtype. The underlying mechanism of the specific enrichment of
F. nucleatum in cecal and ascending colon cancers is still unclear, but microenvironmental or biological factors specifically found in the cecal to ascending colon areas could influence the increase of intratumoral
F. nucleatum. For example, bacterial biofilms are intensively enriched in right-sided colon tumors compared with those in left-sided colorectal tumors [
23]. Based on recent experimental findings, potential molecular mechanisms can be hypothesized. According to a previous experimental study,
F. nucleatum is enriched in colorectal tumor tissue by Fap2 binding to Gal-GalNAc expressed on tumor cells [
24]. Thus, it can be hypothesized that Gal-GalNAc expression on tumor cells might be more upregulated in the right-sided colon than in the left-sided colon. Further investigations are needed to elucidate the biological reason of the preference of invasive
F. nucleatum for right-sided colon cancers.
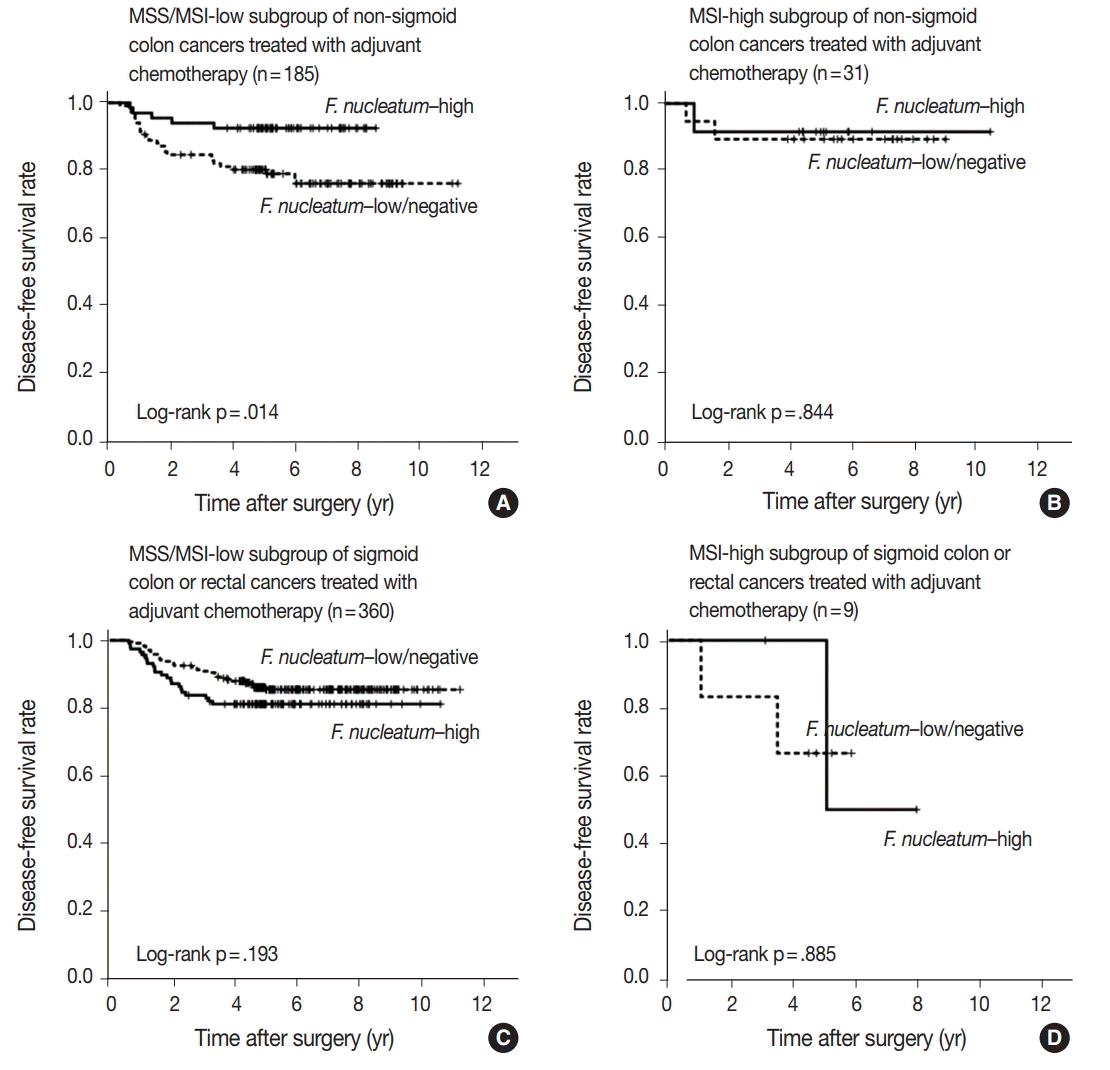
According to the recent data reported by Ogino group,
F. nucleatum in CRCs differentially impacts tumor-infiltrating lymphocyte (TIL) density depending on MSI status [
25]. In detail, there was an inverse association between
F. nucleatum load and TIL density in MSI-high CRCs, whereas a positive correlation between
F. nucleatum load and TIL density was observed in non–MSI-high CRCs [
25]. This finding can provide an important clue for the interpretation of our present results. It has been validated that high TIL density is strongly associated with favorable prognosis in CRCs [
26]. Thus, because
F. nucleatum–high tumors might be associated with increased antitumor immunity and subsequent improved prognosis in non–MSI-high CRCs, the favorable prognostic effect of
F. nucleatum–high in the MSS/MSI-low subset of non-sigmoid colon cancers, which was observed in our present study, could be a reasonable finding. However, we also found that the prognostic significance of
F. nucleatum was valid only in non-sigmoid colon cancers, but not in sigmoid colon/rectal cancers, suggesting that both tumor location and MSI status should be concurrently considered for understanding the prognostic implications of
F. nucleatum in CRCs.
There have been several reports regarding the poor prognostic effect of
F. nucleatum in CRCs, which was mainly observed in Western CRC cohorts or stage IV CRC cohorts [
3,
20,
27]. However, our present data indicate that high intratumoral
F. nucleatum load might be associated with favorable prognosis in a limited subgroup of CRCs, a MSS/MSI-low subset of non-sigmoid colon cancers. We suspect that different compositions of tumor locations and MSI subtypes in CRC cohorts might influence the different prognostic effects of
F. nucleatum in overall CRCs. Because it has been known that the frequency of MSI-high in CRCs is definitely lower in East Asia countries than in Western countries [
28], the potential favorable prognostic effect of
F. nucleatum in proximal colonic-located, non–MSI-high CRCs might be significantly attenuated in CRC cohorts of Western countries, which consist of relatively high numbers of MSI-high tumors. Instead, both the tendency toward worse prognosis of
F. nucleatum–high in MSI-high tumors (
Supplementary Fig. S2A) and the potential poor prognostic effect of
F. nucleatum–high tumors observed in sigmoid colon/rectal cancers (
Fig. 2C) might augment the adverse prognostic impact of
F. nucleatum in overall CRCs. To confirm this hypothesis, additional investigations using various CRC cohorts having different ethnic backgrounds would be needed. Regarding the poor prognostic feature of
F. nucleatum in stage IV CRCs observed in a few studies [
20,
27], it could be explained by relatively high proportion of distal-located CRCs as primary origin of stage IV CRCs. Thus, the potential worse prognostic effect of
F. nucleatum in sigmoid colon or rectal cancers might be augmented especially in a stage IV subset of CRCs.
Although significant associations between CIMP-high (and/or MSI-high) and
F. nucleatum in CRCs were reported in several previous studies [
3,
8,
9], significant correlation between
F. nucleatum–high group and CIMP-high or MSI-high molecular subtype was not observed in our present study (
Table 1). However, there was an evident tendency toward higher proportion of CIMP-high tumors in
F. nucleatum-high group than in
F. nucleatum–low/negative group (7.4% vs. 4.7%) (
Table 1). In addition, we performed mean comparison of
F. nucleatum DNA amount (2-ΔCt) between CIMP-high and CIMP-low/negative tumors, and the results indicated that mean
F. nucleatum DNA amount was higher in CIMP-high tumors than in CIMP-low/negative tumors although statistical significance was not reached (0.986 vs. 0.367, p = .157) (
Supplementary Fig. S3). The reason for unclear molecular association of
F. nucleatum in our study samples may be explained by potential ethnic differences and biased sample composition. As mentioned above, the frequencies of MSI-high and CIMP-high in CRCs are lower in East Asian population than in Western population. If a high number of CIMP-high cases were included in our cohort, significant association between
F. nucleatum–high and CIMP-high might have been observed. Moreover, our study samples were confined to selected stage III or high-risk stage II CRCs treated with adjuvant chemotherapy. Thus, molecular compositions of our CRC cohort were possibly biased. For example, the CIMP-high/non-MSI-high subtype has been known as an aggressive phenotype of CRCs and can be more enriched in stage IV tumors. Because stage IV cases were excluded from our study samples, the potential association between
F. nucleatum–high and CIMP-high could be weakened. Considering that data are limited, the relationship between
F. nucleatum and specific molecular phenotypes in CRCs has not been conclusive yet. Therefore, further clinical and experimental investigations are needed to elucidate whether CIMP-high and/or MSI-high molecular phenotype can significantly interact with intratumoral
F. nucleatum enrichment in CRCs.
The proportion of
F. nucleatum-positive cases in CRCs by qPCR analysis has been variable according to different investigations (8.6%–74%) [
29]. In our results,
F. nucleatum DNA was detected in 408 out of 593 cases (68.8%). The reason for variability in the
F. nucleatum-positive rate in CRCs is unclear, but tissue quality might be a critical factor for this discrepancy. Recently, Lee et al. [
27] found that the tissue fixation method could affect different results of
F. nucleatum qPCR analysis. We also found that when the FFPE tissues were more recent, the positive rate of
F. nucleatum was increased (unpublished data). Therefore, it can be inferred that
F. nucleatum-positive rate by qPCR method could be variable, depending on tissue fixation method and tissue storage time.
There are several limitations in this study. First, we assessed the amount of F. nucleatum in genomic DNA samples extracted from FFPE tissues. The precise quantification of F. nucleatum could be disturbed owing to the degraded nature of DNA extracted from FFPE tissues although a substantial number of previous studies that analyzed F. nucleatum in clinical CRC samples also used FFPE tissue-derived DNA. Second, our study cohort was retrospectively collected. The results from our study should be validated by other prospective studies.
In conclusion, the prognostic impact of F. nucleatum in CRCs treated with adjuvant chemotherapy may differ depending on the combined status of primary tumor location and MSI molecular phenotype. Intratumoral F. nucleatum load may be a potential prognostic factor in stage III or high-risk stage II non-sigmoid colon cancers treated with oxaliplatin-based adjuvant chemotherapy, especially in an MSS/MSI-low molecular subtype. There have been very limited data regarding the detailed prognostic implications of F. nucleatum in CRCs according to various clinicopathologic and molecular contexts. Therefore, further studies using large prospective cohorts will be necessary to validate the different location/MSI-dependent prognostic impacts of F. nucleatum in CRCs treated with adjuvant chemotherapy.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download