Abstract
Background
Vitiligo is a chronic autoimmune disease in which the destruction of melanocytes causes white spots on the affected skin. Janus kinase (JAK) is a family of intracellular, non-receptor tyrosine kinases that transduce cytokine-mediated signals via the JAK–signal transducer and activator of transcription pathway. The aim of the present study is to explore the possible role of JAK1 in the pathogenesis of vitiligo using immunohistochemical methods.
Methods
The current study was conducted in a sample of 39 patients who presented with vitiligo and 22 healthy individuals who were age and sex matched as a control group. We used immunohistochemistry to evaluate JAK1 status (intensity and distribution) and assess the percentage of residual melanocytes using human melanoma black 45 (HMB45).
Vitiligo is a chronic autoimmune disease that results from destruction of melanocytes, causing white spots on the affected skin. Vitiligo affects approximately 1% of people worldwide and can affect both adults and children, causing diminished quality of life and marked psychological distress [1].
Janus kinase (JAK) is a family of intracellular, non-receptor tyrosine kinases that transduce cytokine-mediated signals via the JAK–signal transducer and activator of transcription (STAT) pathway. Approximately 2,000 kinases are known, and more than 90 protein tyrosine kinases (PTKs) have been found in the human genome [2].
The JAK family differs markedly from other classes of PTKs due to the presence of an additional kinase domain. To denote this unique structural feature, these kinases were renamed “Janus kinases” in reference to the ancient two-faced Roman god of gates and doorways. The members of this tyrosine kinase family include JAK1, JAK2, JAK3, and tyrosine kinase 2 [3].
Studies have shown that various cytokines including interferon γ (IFN-γ) [4,5], tumor necrosis factor α [6], and chemokine (C-C motif) ligand 22 [7] are differentially expressed in the lesional skin and serum of vitiligo patients compared to controls, indicating roles in vitiligo. IFN-γ bound receptor complex recruits JAK1 and JAK2 kinases, leading to phosphorylation and nuclear translocation of STAT, which in turn transcriptionally activates downstream IFN-γ–inducible genes. The use of JAK1/3 inhibitors such as tofacitinib may effectively lead to blockade of IFN-γ signaling and downstream CXCL10 expression, thus giving rise to repigmentation in vitiligo [8].
The current study aimed to explore the role of JAK1 in the pathogenesis of vitiligo using immunohistochemical methods.
This prospective case-control study was carried out in a sample of 61 cases, comprising 39 patients who presented with vitiligo and 22 individuals without vitiligo who were age- and sex-matched as a control group. Cases were selected from the Dermatology Outpatient Clinic, Menoufia University Hospital, from February 2017 to July 2017.
Biopsies were performed in 22 apparently healthy age-, sex-, and site-matched normal subjects who were selected as a control group from the Department of Plastic Surgery, Faculty of Medicine, Menoufia University, between February 2017 and July 2017.
A written consent form was approved by the Committee of Human Rights in Research at Menoufia University (443/2018) and obtained from every participant before study initiation.
Exclusion criteria were as follows: (1) patients who received local or systemic treatment before the start of the study; (2) patients who had other autoimmune diseases; and (3) patients less than 18 years of age.
All patients were subjected to the following: complete history including age, sex, onset of disease (younger than 20 years or at and older than 20 years), and disease course assessed by vitiligo disease activity (VIDA) score [9]. Duration of lesion(s) expressed in years, sites, and extension of the lesions and family history of similar conditions were also assessed.
Detailed dermatological examinations were performed to classify types (segmental and nonsegmental) and distribution (acral, acrofacial, focal, vulgaris, segmental, and generalized) of vitiligo.
The patients did not receive any treatment (local or systemic) for at least one month before biopsy. A 3-mm punch biopsy was performed in involved skin of each patient under local anesthesia and in control subjects. Biopsy samples were fixed in neutral formalin 10% and submitted for routine tissue processing in paraffin embedded blocks to the Pathology Department, Faculty of Medicine, Menoufia University. Several 4-μm-thick paraffin embedded sections were cut from each block. One section from each block was stained with hematoxylin and eosin to evaluate pathological changes, while the remaining sections were cut on positive charged slides for immunostaining detection of JAK1 and human melanoma black 45 (HMB45).
Hematoxylin and eosin–stained slides were examined microscopically to evaluate and verify epidermal and dermal pathological changes: (1) evaluation of dermal perivascular inflammatory infiltrate density, divided into mild, moderate and severe; (2) signs of pigmentation in the form of residual melanin in epidermis or dermal melanophages and defined as present or absent.
The method used for immunostaining was a streptavidin-biotin–amplified system. The primary antibodies were rabbit polyclonal antibody against JAK (diluted to 1/100 in antibody diluent; cat. No. Gtx55099, -P1, or –P; 1.0 mL at 100 μg/mL; Genetex, Irvine, CA, USA) and mouse monoclonal antibody directed against HMB45 (ready to use, clone HMB-45, Dako, Copenhagen, Denmark). Slides were subjected to deparaffinization and rehydration. Antigen retrieval was performed by boiling in citrate buffer saline (pH 6), followed by cooling at room temperature. Endogenous perioxidase was blocked by incubation with H2O2, 3%. The primary antibodies were incubated overnight at room temperature, and then the secondary antibody (ready-to-use, Ultravision detection system anti-polyvalent HRP/DAB, Neomarker, Labvision Corp., Fremont, CA, USA) was applied with DAB as a chromogenic substrate and Mayer’s hematoxylin as a counter stain. Human breast cancer was used as a positive control for JAK. Replacement of the primary antibody in the staining procedure with a blocking buffer was included as a negative control.
Positive expression was identified when cytoplasmic expression was seen in any cells. The intensity of expression was evaluated subjectively according to depth of immunostaining as mild (+), moderate (++), and strong (+++). The distribution of staining was diffuse when staining was seen in all epidermal layers and focal otherwise.
Membranous expression in any number of cells was considered positive for HMB45. The percentage of positive cells (melanocytes) in relation to the number of basal keratinocytes was evaluated and expressed as mean, median, and range.
Data were collected, tabulated, and statistically analyzed using a personal computer with SPSS ver. 23 (IBM Corp., Armonk, NY, USA). The chi-square and Fisher exact tests were used for comparisons between qualitative variables. The Mann-Whitney U test and Kruskal-Wallis tests were used for comparisons between quantitative variables. p < .05 was considered significant.
The clinical data for vitiligo patients are presented in Table 1.
JAK1 was expressed in all involved vitiliginous skin (100%), with mild intensity in 18 cases (46.2%) (Fig. 1A), moderate intensity in nine cases (23.1%) (Fig. 1B), and strong intensity in 12 cases (30.8%) (Fig. 1C). There was focal distribution of JAK1 in 21 cases (53.8%) (Fig. 1A) and diffuse expression (Fig. 1A, C) in 18 cases (46.3%). JAK1 expression was mild and exhibited focal distribution in all control samples (Fig. 1D). Only one case of vitiliginous skin showed nuclear and cytoplasmic expression of JAK 1 (Fig. 1B). There was a significant difference in JAK1 expression between vitiliginous and normal skin (p < .001) since intense and diffuse expression was significantly more frequent in vitiliginous skin (Table 2).
JAK1 intensity of expression was associated with disease duration (p = .030), sex (p = .003), presence of melanin pigment (p = .007), and percentage of HMB45 (p = .002). Strong JAK1 expression was associated with short disease duration, female sex, presence of lesional melanin pigment, and lower percentage of HMB45 compared to moderate and mild cases (Table 3). When mild and moderate cases were lumped together versus strong cases by intensity of JAK1, the same correlations were found except for the association with sex (data not shown).
JAK1 distribution (diffuse vs focal) was associated with disease duration (p = .030), sex (p = .020), and percentage of HMB45 (p = .001). Since diffuse expression was associated with short disease duration, female sex, and lower percentage of HMB45 compared to cases with long disease duration, male sex and high percentage HMB45 showed focal expression (Table 4).
The current study demonstrated intense and diffuse JAK1 expression in lesional skin of vitiligo patients compared to controls, where the latter showed mild and focal JAK 1 expression. Our findings agree with those of Nada et al. [10], who found that the level of JAK1 was significantly higher in vitiligo patients than controls. Furthermore, they found that the level of JAK1 in the skin of vitiligo patients after exposure to ultraviolet rays was significantly decreased in comparison to the level before treatment [10]. These findings suggest that JAK1 plays a role in the pathogenesis of vitiligo, and that JAK1 inhibitors may be useful for treatment of vitiligo [10]. JAK inhibitors are reported to delay the onset and reduce the severity of atopic dermatitis-like lesions, resulting in reductions of Th1 and Th2 responses [11].
JAK1 level (intensity and distribution) was associated with sex in vitiligo patients in the present study, as it showed significantly more intense and diffuse expression in females compared to males. No prior studies support or contradict this finding. However, JAKs play important roles in adipose tissue development [12], and females usually have more fatty tissue compared to males, which could explain the high level of JAK in females.
In the current study, we demonstrated that vitiligo cases of short duration were associated with diffuse and intense JAK1 expression compared to cases with prolonged duration. Ineterleukin 17 (IL-17) in patients with vitiligo was previously correlated positively with early age of vitiligo onset and may contribute to immune response in early onset disease through activation by a different pathway [13]. IL-17 activates nuclear factor-kB (NF-κB) and mitogen-activated protein kinase pathways. The adaptor protein NF-κB activator 1 plays an essential role in IL-17–dependent signaling, as well as in activation of JAK1-associated phosphoionositide 3-kinase [14]. On the other hand, positive correlation between JAK1 and long disease duration has been reported in psoriasis, according to Nada et al. (2018) [10], a relationship that highlights the major role of JAK1 in the pathogenesis of psoriasis.
According to the present study, lower percentages of HMB45 were associated with strong and diffuse JAK1 expression in vitiligo lesions. This suggests a role of JAK1 in promoting melanocyte destruction and disappearance. The activation of JAK1 was primarily responsible for transmission of promigration signals that antagonized proliferation and melanogenesis [15]. The association of intense JAK1 expression with the presence of melanin may indicate a role in melanocyte destruction, since melanin was usually present in the dermis due to pigment incontinence descending from the epidermis.
Contradicting our findings, a previous study found that increasing STAT activation was accompanied by up-regulation of JAK, where STATs display significant level of activity in melanocytes and play roles in the survival and growth of melanoma cells [16]. However, Nada et al. (2018) [10] were unable to detect correlations between JAK1 level and clinical and pathological parameters in vitiligo.
Although moderate and strong JAK1 indicated moderate inflammation (Table 3), and diffuse JAK1 expression was more likely in cases of moderate inflammation than focal JAK1 (Table 4), these differences were not significant. This may be due to the limited number of cases in the sample and the absence of cases with intense inflammation
In summary, JAK1 may be involved in the pathogenesis of vitiligo, indicated by its intense and diffuse expression in the skin of vitiligo patients compared to controls and its association with lower percentages of melanocytes detected by HMB45 immunostaining. The association between vitiligo cases of short duration with intense and diffuse JAK1 expression may reflect its immunomodulatory role. Further studies including several clinical types of vitiligo with different VIDA scores are recommended to verify and elucidate the possible role of JAK1 in the etiopathogenesis of vitiligo.
REFERENCES
1. Bonotis K, Pantelis K, Karaoulanis S, et al. Investigation of factors associated with health-related quality of life and psychological distress in vitiligo. J Dtsch Dermatol Ges. 2016; 14:45–9.

2. Bhise SB, Nalawade AD, Wadhawa H. Role of protein tyrosine kinase inhibitors in cancer therapeutics. Indian J Biochem Biophys. 2004; 41:273–80.
3. Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene. 2000; 19:5662–79.

4. Basak PY, Adiloglu AK, Koc IG, Tas T, Akkaya VB. Evaluation of activatory and inhibitory natural killer cell receptors in non-segmental vitiligo: a flow cytometric study. J Eur Acad Dermatol Venereol. 2008; 22:970–6.

5. van den Boorn JG, Konijnenberg D, Dellemijn TA, et al. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol. 2009; 129:2220–32.

6. Attwa E, Gamil H, Assaf M, Ghonemy S. Over-expression of tumor necrosis factor-alpha in vitiligo lesions after narrow-band UVB therapy: an immunohistochemical study. Arch Dermatol Res. 2012; 304:823–30.
7. Klarquist J, Denman CJ, Hernandez C, et al. Reduced skin homing by functional Treg in vitiligo. Pigment Cell Melanoma Res. 2010; 23:276–86.

8. Craiglow BG, King BA. Tofacitinib citrate for the treatment of vitiligo: a pathogenesis-directed therapy. JAMA Dermatol. 2015; 151:1110–2.
9. Bhor U, Pande S. Scoring systems in dermatology. Indian J Dermatol Venereol Leprol. 2006; 72:315–21.

10. Nada HR, El Sharkawy DA, Elmasry MF, Rashed LA, Mamdouh S. Expression of Janus kinase 1 in vitiligo & psoriasis before and after narrow band UVB: a case-control study. Arch Dermatol Res. 2018; 310:39–46.
11. Landry DA, Sormany F, Haché J, Roumaud P, Martin LJ. Steroidogenic genes expressions are repressed by high levels of leptin and the JAK/STAT signaling pathway in MA-10 Leydig cells. Mol Cell Biochem. 2017; 433:79–95.

12. Bassiouny DA, Shaker O. Role of interleukin-17 in the pathogenesis of vitiligo. Clin Exp Dermatol. 2011; 36:292–7.

13. Qian Y, Liu C, Hartupee J, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007; 8:247–56.

14. Alexeev V, Yoon K. Distinctive role of the cKit receptor tyrosine kinase signaling in mammalian melanocytes. J Invest Dermatol. 2006; 126:1102–10.

Fig. 1.
Vitiliginous skin shows mild and focal cytoplasmic staining (A), moderate and diffuse cytoplasmic staining (B), and strong diffuse cytoplasmic and nuclear staining (C). Normal skin shows mild and focal cytoplasmic staining (D).
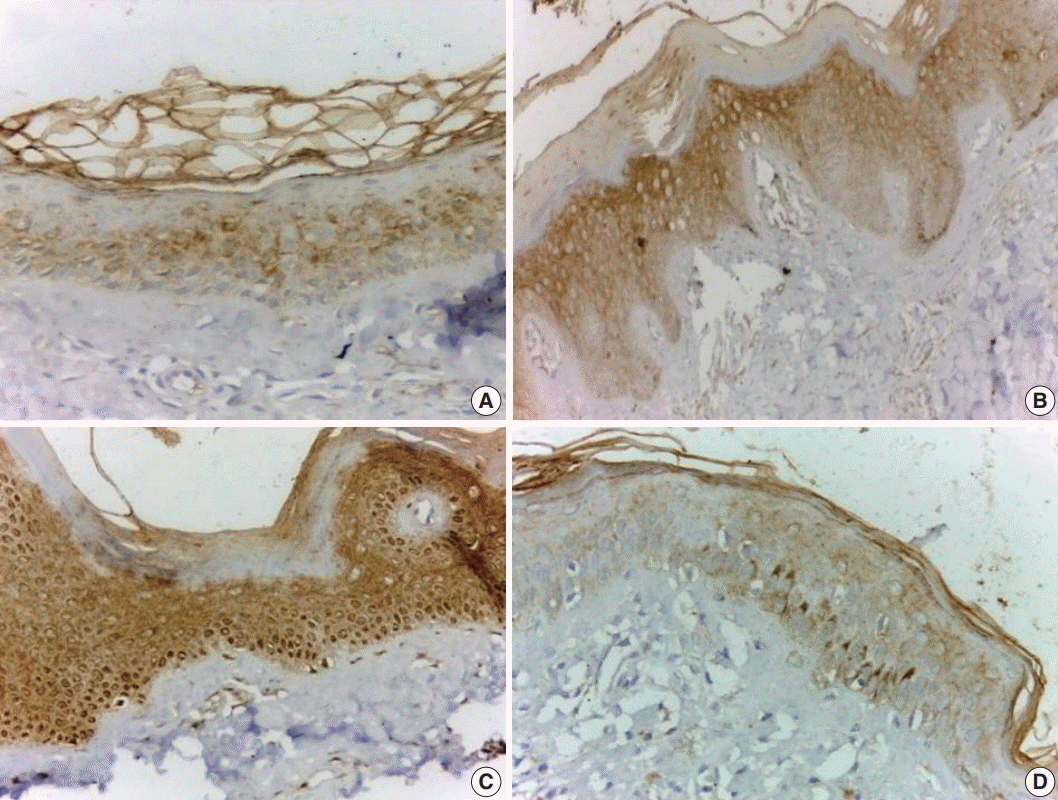
Table 1.
Clinicopathological data of vitiligo patients
Table 2.
JAK1 immunohistochemical expression in the skin of vitiligo patients and controls
Table 3.
The relationships between intensity of JAK1expression and clinicopathological parameters in vitiligo patients
| Clinicopathological parameter | Mild (n = 18) | Moderate (n = 9) | Strong (n = 12) | Statistical test | p-value |
|---|---|---|---|---|---|
| Age (yr) | |||||
| Mean ± SD | 37.00 ± 17.60 | 38.33 ± 12.76 | 29.33 ± 11.57 | 3.48a | .170 |
| Median (range) | 35.00 (18–64) | 33.00 (27–55) | 22.00 (21–45) | ||
| Disease duration (yr) | |||||
| Mean ± SD | 5.67 ± 4.35 | 6.00 ± 3.00 | 3.67 ± 2.46 | 6.53a | .030* |
| Median (range) | 4.00 (3–15) | 4.00 (4–10) | |||
| Sex | 11.44b | .003* | |||
| Male | 12 (66.7) | 0 | 4 (33.3) | ||
| Female | 6 (33.3) | 9 (100) | 8 (66.7) | ||
| Onset (yr) | 4.03b | .130 | |||
| < 20 | 6 (33.3) | 0 | 4 (33.3) | ||
| ≥ 20 | 12 (66.7) | 9 (100) | 8 (66.7) | ||
| Family history | 0.000b | > .999 | |||
| Negative | 12 (66.7) | 6 (66.7) | 8 (66.7) | ||
| Positive | 6 (33.3) | 3 (33.3) | 4 (33.3) | ||
| Distribution | 0.000b | > .999 | |||
| NSV | 6 (33.3) | 3 (33.3) | 4 (33.3) | ||
| SV | 12 (66.7) | 6 (66.7) | 8 (66.7) | ||
| Melanin | 9.85b | .007* | |||
| Absent | 9 (50.0) | 9 (100) | 4 (33.3) | ||
| Present | 9 (50.0) | 0 | 8 (66.7) | ||
| Dermal inflammation | 0.000b | > .999 | |||
| Mild | 12 (66.7) | 6 (66.7) | 8 (66.7) | ||
| Moderate | 6 (33.3) | 3 (33.3) | 4 (33.3) | ||
| HMB45 status | 1.11b | .570 | |||
| Negative | 9 (50) | 3 (33.3) | 4 (33.3) | ||
| Positive | 9 (50) | 6 (66.7) | 8 (66.7) | ||
| HMB45 (%) | 12.20a | .002* | |||
| Mean ± SD | 34.38 ± 34.59 | 6.66 ± 6.61 | 2.5 ± 4.52 | ||
| Median (range) | 30.00 (0–90) | 10.00 (0–15) | 4.52 (0–10) |
Table 4.
The distribution of JAK1 expression and clinicopathological variables in vitiligo patients
| Clinicopathological parameter | Focal (n = 21) | Diffuse (n = 18) | Statistical test | p-value |
|---|---|---|---|---|
| Age (yr) | ||||
| Mean ± SD | 36.43 ± 16.29 | 33.22 ± 13.71 | 0.17a | .860 |
| Median (range) | 33.00 (18–64) | 27.00 (21–55) | ||
| Disease duration (yr) | 2.16a | .030* | ||
| Mean ± SD | 6.29 ± 4.30 | 3.78 ± 1.98 | ||
| Median (range) | 4.00 (3–15) | 4.00 (2–7) | ||
| Sex | 4.88b | .020* | ||
| Male | 12 (57.1) | 4 (22.2) | ||
| Female | 9 (42.9) | 14 (77.8) | ||
| Onset (yr) | 0.21c | .650 | ||
| < 20 | 6 (28.6) | 4 (22.2) | ||
| ≥ 20 | 15 (71.4) | 14 (77.8) | ||
| Family history | ||||
| Negative | 12 (57.1) | 14 (77.8) | 1.85b | .170 |
| Positive | 9 (42.9) | 4 (22.2) | ||
| Distribution | ||||
| NSV | 6 (28.6) | 7 (38.9) | 0.46b | .490 |
| SV | 15 (71.4) | 11 (61.1) | ||
| Melanin | 0.01b | .920 | ||
| Absent | 12 (57.1) | 10 (55.6) | ||
| Present | 9 (42.9) | 8 (44.4) | ||
| Dermal inflammation | ||||
| Mild | 15 (71.4) | 11 (61.1) | 0.46b | .490 |
| Moderate | 6 (28.6) | 7 (38.9) | ||
| HMB45 status | 0.06b | .800 | ||
| Negative | 9 (42.9) | 7 (38.9) | ||
| Positive | 12 (57.1) | 11 (61.1) | ||
| HMB45 (%) | 3.42a | .001* | ||
| Mean ± SD | 30.90 ± 33.18 | 3.33 ± 4.85 | ||
| Median (range) | 15.00 (0–90) | 0 (0–10) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download