Abstract
Background
The aim of this study is to elucidate the clinicopathological significances, including the prognostic role, of metastatic lymph node ratio (mLNR) and tumor deposit diameter in papillary thyroid carcinoma (PTC) through a retrospective review and meta-analysis.
Methods
We categorized the cases into high (≥ 0.44) and low mLNR (< 0.44) and investigated the correlations with clinicopathological parameters in 64 PTCs with neck level VI lymph node (LN) metastasis. In addition, meta-analysis of seven eligible studies was used to investigate the correlation between mLNR and survival.
Results
Among 64 PTCs with neck level VI LN metastasis, high mLNR was found in 34 PTCs (53.1%). High mLNR was significantly correlated with macrometastasis (tumor deposit diameter ≥ 0.2 cm), extracapsular spread, and number of metastatic LNs. Based on linear regression test, mLNR was significantly increased by the largest LN size but not the largest metastatic LN (mLN) size. High mLNR was not correlated with nuclear factor κB or cyclin D1 immunohistochemical expression, Ki-67 labeling index, or other pathological parameters of primary tumor. Based on meta-analysis, high mLNR significantly correlated with worse disease-free survival at the 5-year and 10-year follow-up (hazard ratio [HR], 4.866; 95% confidence interval [CI], 3.527 to 6.714 and HR, 5.769; 95% CI, 2.951 to 11.275, respectively).
Papillary thyroid carcinoma (PTC) is a malignant tumor with favorable prognosis [1]. Neck level VI lymph node (LN) metastasis is associated with primary tumor size, tumor multifocality, and extrathyroidal extension [2]. However, neck level VI LN metastasis is frequently found in up to 80% of patients [3,4]. The prognosis of patients with neck level VI LN metastasis is different from those without nodal disease [5]. The new eighth American Joint Committee on Cancer (AJCC) staging has been introduced and applied in daily practice [6]. An important change in pN stage in the eighth AJCC staging systems is that stage group is not different based on the difference between pN1a and pN1b [6,7]. In the seventh AJCC staging, cases with pN1b disease are defined as stage IVA, regardless of pT stage in patients of 45 years and older [7]. However, in the eighth AJCC staging, pN1 disease is classified as stage II in pT1 and pT2 in 55 years and older patients [6]. Furthermore, pN1 disease is considered as a single group without distinction between pN1a and pN1b in risk assessment. Because the risk assessment models of the eighth AJCC staging are simpler compared with the previous version, a more detailed risk stratification may be necessary.
To predict the prognosis of patients with nodal disease, pathological parameters, such as number of examined LNs and metastatic LNs (mLNs), mLN size, metastatic foci size within mLN, extracapsular spread, and metastatic LN ratio (mLNR), have been introduced and their prognostic roles evaluated [2,6]. The mLNR is defined as the ratio of number of mLNs to number of LNs examined. The prognostic role of the mLNR has been used to study various malignant tumors, including PTCs [8-18]. Positive correlation between high mLNR and worse survival has been reported for various malignant tumors, such as stomach, colorectal, and pancreatic cancers [9,13,15,18-21]. However, detailed criteria for the high mLNR cut-off value and the minimal number of examined LNs required have not been fully elucidated.
The aim of this study is to elucidate the clinicopathological significances of mLNR and the correlations with pathological characteristics of LN through a retrospective review of PTC with neck level VI LN metastasis. In addition, meta-analysis was conducted to evaluate the correlation between high mLNR and survival.
The present study included 122 patients with conventional PTC who received neck level VI LN dissection. All PTCs were surgically resected at Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine (Seoul, Republic of Korea) from January 1 to December 31, 2010. Among 122 patients with PTC, central LN metastasis (level VI) was found in 64. To elucidate the clinicopathological significance of the mLNR, clinicopathological parameters, such as age, sex, tumor size, BRAFV600E mutation, tumor multifocality, and extrathyroidal extension, a review of medical charts and pathological records was performed. All dissected LNs were embedded for microscopic examination, and LN characteristics were evaluated. pTNM staging was performed according to the eighth AJCC TNM classification system [7]. All procedures performed in the current study were approved by the Institutional Review Board (IRB; approval No. KBSMC 2016-04-070). Formal written informed consent was waived by the IRB.
All embedded LNs were evaluated by reviewing glass slides. Evaluated characteristics were number of dissected LNs and mLNs, largest LN size, largest mLN size, and mean LN size, mLNR, and tumor deposit size of mLN. mLNR was defined as number of mLNs to total number of dissected LNs. We categorized the cases into high (≥ 0.44) and low mLNR (< 0.44) and into macrometastasis (tumor deposit size ≥ 0.2 cm) and micrometastasis (< 0.2 cm). In the present study, based on median cut-off value (mLNR, 0.44), eligible studies were divided into high and low mLNR subgroups.
Two array blocks containing 64 PTCs with neck level VI LN metastasis were prepared for immunohistochemistry. A tissue core was obtained from each primary tumor. Core tissue biopsies (2 mm diameter) were collected from individual paraffin-embedded donor PTC blocks and delivered in recipient tissue array blocks using a trephine apparatus. Each block contained nonneoplastic thyroid tissue as an internal control.
Sections 4 μm in size were cut, deparaffinized, and hydrated using routine xylene-alcohol procedures before incubation with 0.01 M citrate buffer (pH 6.0) for 5 minutes in a microwave oven for antigen retrieval and treatment with 3% H2O2 to quench endogenous peroxidase. Sections were treated with normal serum from the animal used to generate secondary antibodies to block nonspecific binding and then incubated with anti-nuclear factor κB (NF-κB) RelA (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-cyclin D1 (1:100, Thermo Fisher Scientific, Fremont, CA, USA), or anti-Ki-67 (1:50, Santa Cruz Biotechnology). A compact polymer method from Bond Intense detection kits (Leica Biosystems, Newcastle upon Tyne, UK) was used for immunohistochemical staining and 3,3'-diaminobenzidine (Vector Laboratories, Burlingame, CA, USA) for visualization. To confirm reaction specificity, a negative control without primary antibody was included. For counterstaining, immunostained sections were lightly stained with Mayer’s hematoxylin.
Nuclear expression of NF-κB RelA, regardless of cytoplasmic expression, was considered as NF-κB activation. In the present study, NF-κB positivity was defined as NF-κB stain in ≥ 5% of tumor cell nuclei [22-24]. Regarding interpretation for cyclin D1, patients showing nuclear staining in κ 10% of the tumor cells were considered positive [25]. For Ki-67 staining, we evaluated at least 300 cells and counted cells with nuclear staining for each specimen. Proliferation index (%) was 100 × Ki-67-positive cells/300 cells.
Relevant articles were obtained by searching PubMed and MEDLINE databases up to May 15, 2018. Searches were performed using the keywords ‘papillary thyroid carcinoma’ and ‘metastatic lymph node ratio or lymph node ratio.’ The title and abstract of all searched articles were screened for exclusion. Review articles were also screened to find additional eligible studies. Search results were then scanned based on the following inclusion and exclusion criteria: (1) human thyroid tissue was investigated, (2) available information for the correlation between mLNR and survival in PTC with neck level VI LN metastasis, (3) case reports or non-original articles were excluded, and (4) non-English language publications were excluded. Data from all eligible studies were extracted by two authors. The following data were extracted from each of the eligible studies [8,10-12,14,16,26]: first author’s name, year of publication, number of patients analyzed, and correlation between mLNR and survival based on previous studies [27,28].
SPSS ver. 22.0 software (IBM Corp., Armonk, NY, USA) was used for statistical analyses. The correlations between mLNR and clinicopathological parameters, including the immunohistochemical results of primary tumor, were determined using either chi-square test or the Fisher exact test (two-sided). The relationships between mLNR and tumor size, LN size, mLN size, and tumor deposit diameter of LN were analyzed using a two-tailed Student’s t-test. Linear regression analysis was conducted to investigate correlations between primary tumor size and tumor multifocality, number of tumors, and supplemental tumor size.
In the meta-analysis, data were analyzed using the Comprehensive Meta-Analysis software package (Biostat, Englewood, NJ, USA). The correlation between high mLNR and survival rate in patients with PTC was investigated using meta-analysis. We performed subgroup analysis based on follow-up period and cut-off value for high mLNR. Heterogeneity between studies was analyzed using the Q and I2 statistics and presented as p-values. In addition, sensitivity analysis was conducted to assess the heterogeneity of eligible studies and the impact of each study on the combined effect. Because various cut-off values for high mLNR were used for various populations, the application of random-effect model rather than fixed-effect model was more suitable. The assessment of publication bias was performed using Begg’s funnel plot and Egger’s test. The results were considered statistically significant at p < .05.
Among 122 patients with PTCs, neck level VI LN metastasis was found in 64. We first investigated the clinicopathological significance of high mLNR (≥ 0.44) in 64 PTC patients with neck level VI LN metastasis. No significant correlation was observed between high mLNR and clinicopathological parameters, such as age, sex, tumor size, BRAFV600E mutation, tumor multifocality, and pTNM stage (Table 1). Next, the correlations between mLNR and pathological statuses of examined and mLNs were evaluated. The tumor deposit diameters of mLNs were 0.43 ± 0.31 cm and 0.29 ± 0.41 cm in high and low mLNR, respectively (p = .114) (Table 2). The rate of macrometastasis (> 0.2 cm) in high mLNR was significantly higher than in low mLNR (82.9% vs 44.8%, p = .006). In addition, extracapsular spread of LN was more frequently found in high mLNR than in low mLNR (37.1% vs 6.9%, p = .007). However, high mLNR was not significantly correlated with mean size or largest size of examined and mLNs. The mLNR was significantly increased by the largest LN size, but not the largest mLN size (p = .001 vs p = .364) (Fig. 1). In the high mLNR group, the largest examined LN and the largest mLN were equal to 94.1%; however, in the low mLNR group, the rate was 56.7%.
We previously reported that nuclear NF-κB RelA of the primary tumor significantly correlated with LN metastasis [29]. In the present study, the correlations between mLNR and the immunohistochemical expressions of primary tumor, such as NF-κB RelA, cyclin D1, and Ki-67 labeling index, were investigated. High mLNR was not significantly correlated with nuclear NF-κB RelA (p = .271) or cyclin D1 expression (p > .999). In addition, no significant correlation between high mLNR and ki-67 labeling index was observed (p = .917).
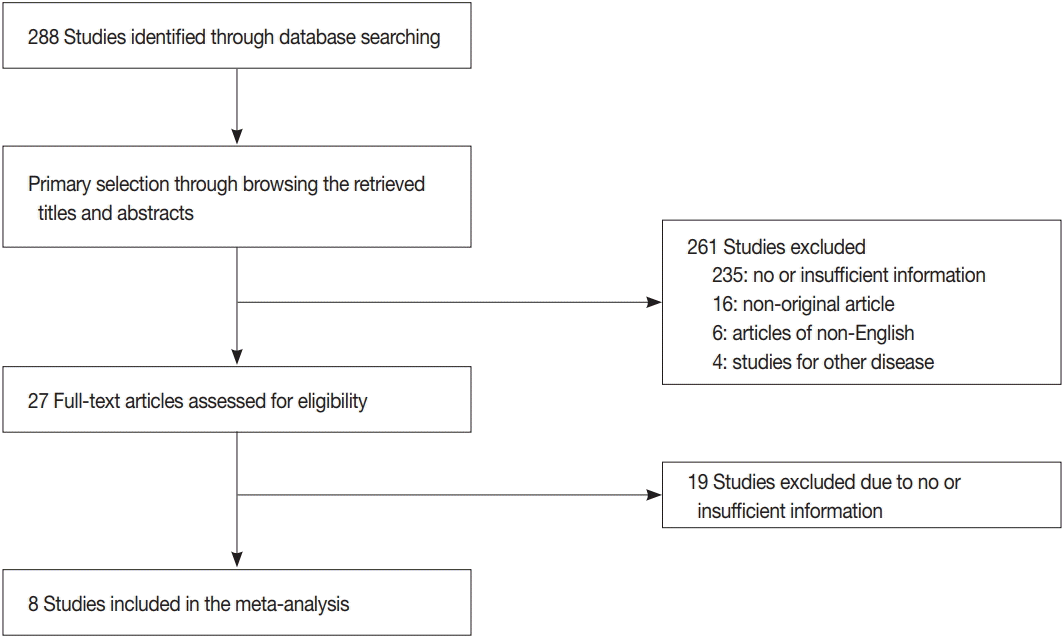
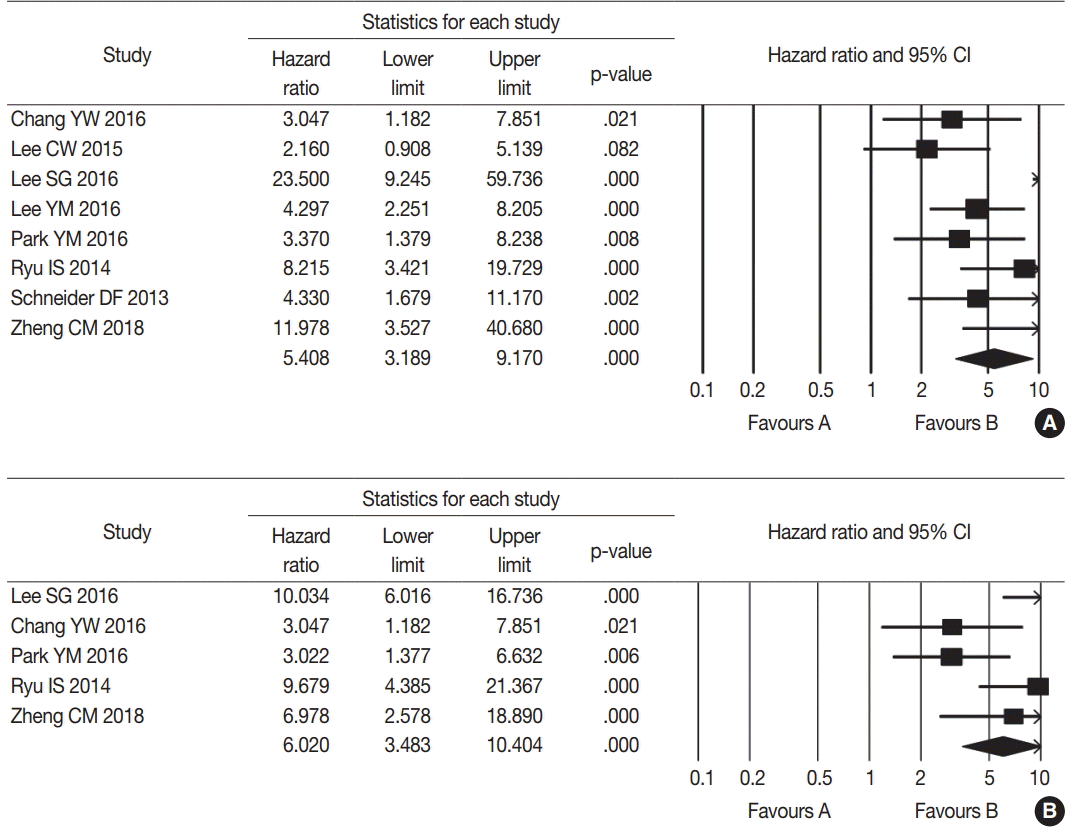
The database search identified 288 reports. Of these, 254 reports were excluded due to lack of sufficient information. Other studies were excluded because they reported the results of other diseases (n = 4), were non-English (n = 6), or were non-original articles (n = 16) (Fig. 2). After applying the inclusion and exclusion criteria, seven reports were finally included for meta-analysis [8,10-12,14,16,26]. Eligible studies used various cut-off values for high mLNR ranging from 0.22 to 0.65. To elucidate the prognostic role of mLNR, the correlation between high mLNR and survival rate was investigated. High mLNR significantly correlated with worse disease-free survival at the 5-year and 10-year follow-up (hazard ratio [HR], 5.408; 95% confidence interval [CI], 3.189 to 9.170 and HR, 6.020; 95% CI, 3.483 to 10.404, respectively) (Fig. 3). To evaluate the optimal cut-off value for high mLNR, subgroup analysis based on a cut-off value of 0.44, was performed. High (≥ 0.44) and low mLNR cut-off (< 0.44) subgroups showed significant correlation between high mLNR and worse disease-free survival at the 5-year follow-up (HR, 5.088; 95% CI, 1.926 to 13.443 and HR, 5.570; 95% CI, 2.833 to 10.952; respectively) and 10-year follow-up (HR, 5.597; 95% CI, 1.806 to 17.350 and HR, 6.158; 95% CI, 2.863 to 13.245, respectively).
Involvement of neck level VI LN is common in PTC and occurs in up to 80% of patients [29]. The clinicopathological significance of neck level VI LN metastasis is of less concern because patients with PTC have favorable prognosis. The nodal disease is subdivided into pN1a and pN1b based on the location of involved LNs in the eighth AJCC staging [6]. However, usefulness of pN1a disease stratification for predicting prognosis has not been fully elucidated. In the present study, the correlations between mLNR and pathological features of mLN and the prognostic role of mLNR in patients with PTC were evaluated using a retrospective study and meta-analysis.
In AJCC staging, pN stage is subdivided into pN1a and pN1b based on the location of the involved nodes in PTC. If involvement of neck level VI LN is identified without lateral neck LN involvement, pN stage is defined as pN1a. In a previous study, PTC with pN1a showed worse prognosis compared with pN0, regardless of pT stage [5]. However, because higher rate of neck level VI LN metastasis is well known, stratification of pN1a may be needed for predicting the detailed prognosis of patients with nodal disease. Various parameters, such as numbers of examined and mLNs, mLN size, metastatic foci size within mLNs, extracapsular spread, and mLNR, have been introduced [2,6]. However, the prognostic role of various parameters has not been fully understood. In addition, the detailed criteria for evaluation of various parameters are neither elucidated nor introduced in the eighth AJCC staging [6].
The introduction of mLNR is included in the eighth AJCC staging for various malignant tumors, but application of pN stage has not been implemented [6]. Although the number of reports on the prognostic role of mLNR in various malignant tumors is increasing [9,13,15,18-21], conclusive information regarding the mLNR in PTCs is not available. The definition of mLNR is the ratio of number of mLNs to number of examined LNs. Therefore, the number of examined LNs can affect the mLNR. In colon cancer, positive LNs were increased based on harvested LNs [30]. In addition, to evaluate the prognostic role of mLNR, the minimal number of examined LNs is required. However, the pertinent cut-off values for examined LNs have not been fully evaluated in PTC. In the eighth AJCC staging, the criteria for examined or mLN numbers has not been determined when evaluating pN stage.
In a previous study, the pathological characteristics of primary tumor showed an effect on LN metastasis [29]. However, in the present study, high mLNR was not significantly correlated with characteristics of the primary tumor. To date, the correlations between mLNR and pathological features of examined and mLNs have not been fully understood. The present study showed that high mLNR significantly correlated with macrometastasis (> 0.2 cm), extracapsular spread, and largest LN size. However, significant correlation between high mLNR and largest or mean mLN size was not observed. On preoperative ultrasonography, several features such as node size, hyperechogenicity, echogenicity of hilum, calcification, and intranodal cystic necrosis, indicated suspicion of metastasis [29]. These are not absolute criteria because LNs detected on preoperative ultrasonography might not match postoperative pathological examinations [31]. Therefore, the prediction of mLNR is preoperatively difficult before pathologic examination.
In a previous study using the Surveillance, Epidemiology, and End Results (SEER) Registry Data, high mLNR was not significantly correlated with worse overall survival (HR, 0.95; 95% CI, 0.58 to 1.57) [32]. However, because this report may have included PTCs with lateral neck LN metastasis, the prognostic role of mLNR in PTC with pN1a disease cannot be evaluated. To date, the prognostic role of high mLNR in PTC with pN1a disease has not been fully investigated due to higher incidence and relatively better prognosis of pN1a disease in patients with PTC. In addition, usefulness of prognostic stratification in pN1a disease is unclear. In the current meta-analysis, high mLNR significantly correlated with worse disease-free survival. Based on our results, the prognostic stratification of pN1a is possible. Compared to other pathological parameters, mLNR is advantageous due to its simplicity and objectivity and lack of interobserver difference. In a previous study, the prognosis was different between patients with pN1a and pN0 disease [5]. However, prognosis may be controversial based on the follow-up period. Our results showed that, regardless of follow-up period, mLNR was a predictor of worse prognosis in PTC with pN1a disease.
Eligible studies used various high mLNR cut-off values ranging from 0.22 to 0.65. In the current meta-analysis, subgroup analysis showed that high (≥ 0.44) and low mLNR cut-off (< 0.44) subgroups showed a correlation between high mLNR and worse disease-free survival at the 5-year and 10-year follow-up. Among eligible studies in the low mLNR cut-off subgroup (< 0.44), Lee et al. [10] showed no significant correlation between high mLNR and worse disease-free survival at 5-year follow-up, regardless of higher HR (> 1.0). In the current meta-analysis, the optimal cut-off value could not be determined due to similar results obtained in the high and low mLNR cut-off subgroups. Additional studies are necessary to define the optimal cut-off value; however, the prognostic role of mLNR in PTC with pN1a disease was demonstrated, regardless of mLNR cut-off value.
In conclusion, our results showed that high mLNR significantly correlated with macrometastasis and extracapsular spread of mLNs. In addition, a significant correlation between high mLNR and worse disease-free survival was found in meta-analysis. Therefore, in situations with simplified pN stage, mLNR may be important for prediction and stratification of the prognosis of patients with nodal disease. Further cumulative studies for the optimal high mLNR cut-off value and the minimal number of examined LNs required are needed before application in daily practice.
REFERENCES
1. DeLellis RA, Lloyd RV, Heitz PU, Eng C. World Health Organization classification of tumours. Pathology and genetics of tumours of endocrine organs. 4th ed. Lyon: International Agency for Research on Cancer;2017.
2. Pyo JS, Sohn JH, Kang G, Kim DH, Kim DH. Characteristics of neck level VI lymph nodes in papillary thyroid carcinoma: correlation between nodal characteristics and primary tumor. Endocr Pathol. 2015; 26:15–20.

4. Sugitani I, Fujimoto Y, Yamada K, Yamamoto N. Prospective outcomes of selective lymph node dissection for papillary thyroid carcinoma based on preoperative ultrasonography. World J Surg. 2008; 32:2494–502.

5. Ito Y, Miyauchi A, Jikuzono T, et al. Risk factors contributing to a poor prognosis of papillary thyroid carcinoma: validity of UICC/AJCC TNM classification and stage grouping. World J Surg. 2007; 31:838–48.

6. Amin MB, Edge SB, Greene FL, et al. The AJCC cancer staging manual. 8th ed. New York: Springer-Verlag;2016.
7. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer-Verlag;2009.
8. Chang YW, Kim HS, Jung SP, et al. Pre-ablation stimulated thyroglobulin is a better predictor of recurrence in pathological N1a papillary thyroid carcinoma than the lymph node ratio. Int J Clin Oncol. 2016; 21:862–8.

9. Inoue K, Nakane Y, Iiyama H, et al. The superiority of ratio-based lymph node staging in gastric carcinoma. Ann Surg Oncol. 2002; 9:27–34.

10. Lee CW, Roh JL, Gong G, et al. Risk factors for recurrence of papillary thyroid carcinoma with clinically node-positive lateral neck. Ann Surg Oncol. 2015; 22:117–24.

11. Lee SG, Ho J, Choi JB, et al. Optimal cut-Off values of lymph node ratio predicting recurrence in papillary thyroid cancer. Medicine (Baltimore). 2016; 95:e2692.

12. Lee YM, Sung TY, Kim WB, Chung KW, Yoon JH, Hong SJ. Risk factors for recurrence in patients with papillary thyroid carcinoma undergoing modified radical neck dissection. Br J Surg. 2016; 103:1020–5.

13. Leonard D, Remue C, Abbes Orabi N, et al. Lymph node ratio and surgical quality are strong prognostic factors of rectal cancer: results from a single referral centre. Colorectal Dis. 2016; 18:O175–84.

14. Park YM, Wang SG, Shin DH, Kim IJ, Son SM, Lee BJ. Lymph node status of lateral neck compartment in patients with N1b papillary thyroid carcinoma. Acta Otolaryngol. 2016; 136:319–24.

15. Rodríguez Santiago JM, Muñoz E, Martí M, Quintana S, Veloso E, Marco C. Metastatic lymph node ratio as a prognostic factor in gastric cancer. Eur J Surg Oncol. 2005; 31:59–66.

16. Ryu IS, Song CI, Choi SH, Roh JL, Nam SY, Kim SY. Lymph node ratio of the central compartment is a significant predictor for locoregional recurrence after prophylactic central neck dissection in patients with thyroid papillary carcinoma. Ann Surg Oncol. 2014; 21:277–83.

17. Schneider DF, Mazeh H, Chen H, Sippel RS. Lymph node ratio predicts recurrence in papillary thyroid cancer. Oncologist. 2013; 18:157–62.

18. Sugimoto K, Sakamoto K, Tomiki Y, Goto M, Kojima Y, Komiyama H. The validity of predicting prognosis by the lymph node ratio in node-positive colon cancer. Dig Surg. 2013; 30:368–74.

19. Opfermann KJ, Wahlquist AE, Garrett-Mayer E, Shridhar R, Cannick L, Marshall DT. Adjuvant radiotherapy and lymph node status for pancreatic cancer: results of a study from the Surveillance, Epidemiology, and End Results (SEER) Registry Data. Am J Clin Oncol. 2014; 37:112–6.

20. Partelli S, Fernandez-Del Castillo C, Bassi C, et al. Invasive intraductal papillary mucinous carcinomas of the pancreas: predictors of survival and the role of lymph node ratio. Ann Surg. 2010; 251:477–82.
21. Pawlik TM, Gleisner AL, Cameron JL, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007; 141:610–8.

22. Pacifico F, Leonardi A. NF-kappaB in solid tumors. Biochem Pharmacol. 2006; 72:1142–52.
23. Pyo JS, Ko YS, Kim WH, et al. Impairment of nuclear factor-kappaB activation increased glutamate excitotoxicity in a motoneuron-neuroblastoma hybrid cell line expressing mutant (G93A) Cu/Zn-superoxide dismutase. J Neurosci Res. 2010; 88:2494–503.
24. Kim B, Byun SJ, Kim YA, et al. Cell cycle regulators, APC/betacatenin, NF-kappaB and Epstein-Barr virus in gastric carcinomas. Pathology. 2010; 42:58–65.
25. Lee KH, Lee HE, Cho SJ, et al. Immunohistochemical analysis of cell cycle-related molecules in gastric carcinoma: prognostic significance, correlation with clinicopathological parameters, proliferation and apoptosis. Pathobiology. 2008; 75:364–72.

26. Zheng CM, Ji YB, Song CM, Ge MH, Tae K. Number of metastatic lymph nodes and ratio of metastatic lymph nodes to total number of retrieved lymph nodes are risk factors for recurrence in patients with clinically node negative papillary thyroid carcinoma. Clin Exp Otorhinolaryngol. 2018; 11:58–64.

27. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998; 17:2815–34.

28. Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985; 27:335–71.

29. Pyo JS, Kang G, Kim DH, et al. Activation of nuclear factor-kappaB contributes to growth and aggressiveness of papillary thyroid carcinoma. Pathol Res Pract. 2013; 209:228–32.
30. Vaccaro CA, Im V, Rossi GL, et al. Lymph node ratio as prognosis factor for colon cancer treated by colorectal surgeons. Dis Colon Rectum. 2009; 52:1244–50.

31. Yang K, Wang H, Liang Z, Liang J, Li F, Lin Y. BRAFV600E mutation associated with non-radioiodine-avid status in distant metastatic papillary thyroid carcinoma. Clin Nucl Med. 2014; 39:675–9.
Fig. 1.
Correlation between metastatic lymph node ratio (mLNR) and largest lymph node size (A) and largest metastatic lymph node (mLN) size (B) based on linear regression.
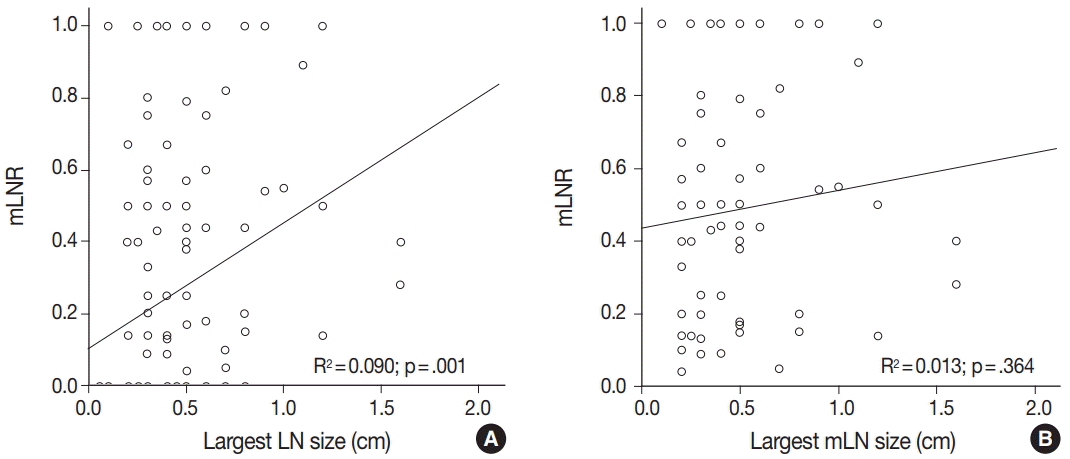
Table 1.
The correlation between mLNR and clinicopathological features in PTCs
Table 2.
The correlation between mLNR and pathological status of LN in papillary thyroid carcinomas




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download