Abstract
Background
Primary endobronchial smooth muscle tumors (SMTs), which are extremely rare, include endobronchial leiomyomas and leiomyosarcomas. Clinically, SMTs present with signs and symptoms of bronchial obstruction, and lack specific radiological findings. Thus, histopathological examination is required for accurate diagnosis as well as for tumor grading. We examined the histomorphological and immunohistochemical features of endobronchial SMTs and highlighted pitfalls in diagnosis, particularly when using small biopsies.
Methods
Cases of primary endobronchial SMTs diagnosed at our Institute over the last 6 years (2012–2017) were retrieved from the departmental archives. Histopathological features and immunohistochemistry performed for establishing the diagnosis were reviewed.
Results
Five cases of SMTs occurring in endobronchial locations were identified. These included three cases of leiomyoma, and two cases of leiomyosarcoma. The age distribution of patients ranged from 13 to 65 years. Leiomyomas showed more consistent staining with smooth muscle markers (smooth muscle actin, desmin, and smooth muscle myosin heavy chain), while tumors of higher grade showed variable, focal staining, leading to erroneous diagnosis, especially on small biopsies.
Conclusions
The diagnosis of endobronchial SMTs relies on histopathological examination, for both confirmation of smooth muscle lineage and determination of the malignant potential of the lesion. Appropriate immunohistochemical panels including more than one marker of smooth muscle differentiation are extremely valuable for differential diagnosis from morphological mimics, which is necessary for instituting appropriate management.
Primary bronchopulmonary mesenchymal tumors are rare. Among these, the most frequent are smooth muscle tumors (SMTs), which include leiomyomas and leiomyosarcomas (LMS) [1]. They are believed to arise from the peri-bronchiolar/interstitial smooth muscle, or rarely from smooth muscle of arteriolar walls [2]. Leiomyomas constitute 2% of benign lung tumors, and are more commonly located in lung parenchyma (51%), while endobronchial and tracheal leiomyomas account for 33% and 16%, respectively [2]. LMS accounts for less than 0.5% of primary pulmonary malignancies, and approximately one-third of primary sarcomas of the lung [3]. Only a few cases of endobronchial localization of LMS have been reported in the literature [3].
Patients with endobronchial SMTs present with symptoms reflecting partial or complete obstruction of the affected bronchus, including wheezing, orthopnea, hemoptysis, or changes in the lung distal to the obstruction, such as recurrent pneumonia and subsequent bronchiectasis [4]. SMTs have been observed in patients with human immunodeficiency virus infections, as well as other immunosuppressed conditions. Coinfection with Epstein-Barr virus (EBV) may play a role in the development of these tumors. AIDS-related EBV-associated SMTs have been reported in endobronchial locations as well [5]. Imaging per se does not help much in the diagnosis of SMTs as there are no pathognomonic features to differentiate SMTs from more common endobronchial tumors [4]. Thus, histopathological examination is required for accurate diagnosis as well as for tumor grading, as the prognosis of SMTs depends upon the grade and degree of differentiation of the tumor. Complete surgical resection is the favored treatment modality for SMTs, irrespective of tumor grade. We describe five cases of endobronchial SMTs and their clinicoradiopathological features, and highlight difficulties in differential diagnosis.
Cases of primary endobronchial SMTs diagnosed at our institution over a period of 6 years (2012–2017) were retrieved from the departmental archives. Clinical, radiological, and treatment details were collected from the hospital medical record system and by telephonic conversation. Hematoxylin and eosin stained slides were retrieved, and histopathological features, as well as immunohistochemistry performed for establishing the diagnosis, were reviewed. Immunostaining for EBV–latent membrane protein 1 (LMP1) was performed in all cases. Approval from the Institute Ethics Committee (IEC no. 404/02.09.2016), All India Institute of Medical Sciences, was obtained to conduct this retrospective study on archival patient samples and thus, informed consent was waived due to the retrospective nature of this study.
Five cases of SMTs occurring in endobronchial locations were identified from the records, including one previously published case [6]. These included three cases of leiomyoma and two cases of LMS. In all cases, the tumor was excised through transtracheal or transbronchial approaches. Clinical presentation, radiological findings, and clinical diagnoses are summarized in Table 1. Patient 1 underwent bronchoscopy (Fig. 1) and biopsy at our institution, after which a diagnosis of leiomyoma was made. He subsequently underwent a lobectomy at a private hospital. Patient 4 underwent a biopsy, after which a diagnosis of inflammatory myofibroblastic tumor (IMT) was made. This was followed by excision of the tumor repeated thrice due to the presence of residual disease. For patient 5 only a biopsy was performed, as she presented with an endobronchial mass with multiple metastases to the bone and to both lobes of the liver, suggesting a primary in an endobronchial location. Detailed clinical workups did not reveal any evidence of primary extrapulmonary malignancy in any of the cases.
Histopathological examination showed squamous metaplasia of the lining bronchial epithelium in cases 1 and 2. The subepithelium showed a sparsely cellular spindle cell tumor with cells arranged in short and long intersecting fascicles (Fig. 2A). The cells had abundant eosinophilic cytoplasm, and ovoid nuclei with homogeneous chromatin (Fig 2B). Paranuclear vacuoles were noted focally. The cells did not show nuclear atypia or mitotic figures; necrosis was also absent. Case 1 showed few inflammatory cells, including lymphocytes and histiocytes, interspersed between tumor cells. Areas of myxoid change, hyalinization, calcification, and mast cells were noted in case 2 (Fig. 2C, D). Immunohistochemical features are summarized in Table 2.
Microscopic examination (Figs. 3, 4) showed tissue fragments with metaplastic squamous epithelial lining. The subepithelium revealed the presence of cellular spindle cell tumor, with cells arranged in short and long intersecting fascicles. The tumor cells were plump with moderate amounts of pale to brightly eosinophilic cytoplasm, had ovoid to elongated vesicular nuclei with small conspicuous nucleoli, and occasionally demonstrated paranuclear vacuoles. There was moderate nuclear pleomorphism, and a mitotic count of 6–7/10 high-power field (HPF) in case 5. A few hyalinized blood vessels and focal myxoid changes were also noted. The first biopsy in case 4 revealed mostly fibrin, acute inflammatory exudate, and a tiny fragment from a spindle cell tumor displaying moderate nuclear pleomorphism, with prominent nucleoli, and an occasional mitotic figure (Fig. 3A, B). The presence of many interspersed inflammatory cells led to a diagnosis of IMT, a more common mesenchymal neoplasm in this location, although anaplastic lymphoma kinase 1 was negative. Paranuclear vacuoles were not evident in the initial biopsy, obscuring the correct diagnosis. Subsequent excision biopsies (Fig. 3C–F) showed a cellular spindle cell tumor with similar tumor morphology to case 5. In addition, there was moderate to marked nuclear pleomorphism, with pleomorphic tumor giant cells intimately admixed with a complement of more uniformappearing spindle cells. Few eosinophils, mast cells, and plasma cells were also identified scattered among the tumor cells. Mitotic figures varied from 1–2/10 HPF. Necrosis was not seen in either of the cases. Both cases showed variable immunostaining patterns, even in separate biopsies from the same patient (Table 2).
The supporting fibroconnective tissues of the trachea and bronchi can give rise to a variety of benign and malignant mesenchymal tumors. However, these are far less common than epithelial tumors [7]. Primary SMTs of the respiratory tract are extremely rare, with leiomyomas comprising approximately 2% of benign lung tumors [7] and LMS accounting for less than 0.5% of malignant primary lung tumors. However, LMS is the most frequent primary lung sarcoma [3]. SMTs can occur along the tracheo-bronchial tree, or within the lung parenchyma [4]. Approximately onethird of pulmonary leiomyomas have endobronchial locations [2]. Most primary pulmonary LMSs are intraparenchymal, with direct extension to the bronchi giving rise to an endobronchial component [7]. The primary endobronchial form of LMS is exceptional, with only 16 adult cases having been documented to date [3,8]. Tracheobronchial leiomyomas occur most commonly in the fourth decade of life, although one-third of reported patients are younger than 20 years of age [9], and affect both sexes equally [10]. LMSs also occur in middle aged patients, but with a slight male predominance [3]. In our series, the patients demonstrated wide age distributions of 13 to 65 years for leiomyoma and 28 to 57 years for LMS. Contrary to published data [3,10], leiomyomas were seen only in males, and LMS only in female patients in our series.
Presenting symptoms of endobronchial SMTs are based on the degree of bronchial obstruction, with the most common being coughing, followed by hemoptysis and wheezing [10]. Radiologically, atelectasis is the most frequent finding, although normal imaging, solitary round mass, pneumonic infiltration, unilateral emphysema, and hyperlucency due to air trapping distal to the obstructed bronchus may also be seen on chest radiographs. Calcification has also been reported in leiomyomas [4], as seen in one of our cases. Definitive diagnosis can be achieved by direct visualization of the lesion by bronchoscopy, followed by biopsy and histopathological characterization [2]. As metastatic pulmonary sarcomas are more common than primary sarcomas, it is necessary to rule out metastases from extrapulmonary sites before establishing a diagnosis of primary pulmonary LMS. Similarly, the possibility of a benign metastasizing leiomyoma should be excluded by the absence of previous surgical history or radiological evidence of a mass at any other site, as done in all our cases.
Differentiation in pulmonary LMS can range from low, intermediate, and high grade. Low-grade tumors recapitulate smooth muscle cell differentiation with low mitotic rates (< 3/10 HPF) as well as absence of cellular atypia, necrosis, and hemorrhage. Intermediate-grade tumors have increased cellularity, with mild to moderate nuclear atypia, and brisk mitotic activity (3–8/10 HPF). High-grade LMS show marked increases in cellularity, nuclear pleomorphism, high mitotic activity (average 8–12 mitoses/10 HPF), abundant necrosis, and hemorrhaging [11]. Primary pulmonary LMS can show a wide spectrum of differentiation with presence of necrosis, cytological atypia, and mitoses [1]. Well-defined criteria for these tumors at endobronchial locations have not been described, and therefore the diagnoses in our cases were based on the criteria discussed above. Diagnoses of intermediate grade LMS of were rendered on the basis of increased cellularity and nuclear pleomorphism in case 4, and increased mitotic activity in case 5.
Upon immunohistochemistry, benign and low grade malignant tumors are more likely to show immunoreactivity for smooth muscle markers, such as smooth muscle actin (SMA), h-caldesmon, and desmin, while high-grade or less differentiated tumors may be negative for all muscle markers, and may require ultrastructural examination to confirm the diagnosis [11]. In our cases as well, leiomyomas showed more consistent staining with smooth muscle markers (SMA, desmin, and smooth muscle myosin heavy chain), while LMSs showed variable, focal staining, leading to erroneous diagnoses, especially on small biopsies.
Several benign and malignant neoplasms enter the differential diagnosis, and can be distinguished by appropriate immunohistochemical stains. They include spindle cell or sarcomatoid carcinoma (positive for epithelial markers), IMT (less cellular, with collagen- rich stroma, prominent plasma cell infiltration, and ALK1 positivity in approximately 50% of cases), monophasic spindle cell synovial sarcoma (CD99 positive), malignant peripheral nerve sheath tumor (S-100 positive), gastrointestinal stromal tumor (CD117 and DOG1 positive), and spindle cell melanoma (human melanoma black 45 positive). The use of more than one smooth muscle marker is recommended in differential diagnostic immunohistochemical panels due to such variable staining, particularly in high grade malignant SMTs.
SMTs arising in immunocompetent patients are not associated with EBV [5]. None of the patients included in this study had a history of AIDS or immunosuppression. On immunohistochemistry, all cases were immunonegative for EBV-LMP1, thus excluding diagnoses of EBV-associated SMT.
Bronchoscopic interventions have shown good results for the resection of endobronchial leiomyoma, and a variety of techniques like electrocautery, argon plasma coagulation, cryotherapy, and Nd:YAG laser can be used for this purpose [12]. The prognosis of these tumors is excellent after complete resection, although rare recurrences have been documented in the literature [13,14]. Kim et al. [9] reported that respiratory tract leiomyomas may show an iceberg growth pattern with both intra- and extra-luminal tumor components, and bronchoscopic removal of the visible intraluminal part only may be responsible for local recurrences.
The prognosis of LMS depends on tumor grade and degree of differentiation [11]. Other variables such as size of the tumor, stage at presentation, and completeness of resection also affect survival [12]. The only curative therapy for primary LMS of the lung is radical resection. However, sleeve lobectomy, pneumonectomy, or carinal resection may be necessary to complete resection and prolong survival, depending on the anatomic location and size of the tumor [8]. Survival following excision of these tumors is better than that for a primary lung carcinoma [15]. Chemotherapy has been suggested in cases with metastases; however, it lacks efficacy, resulting in only partial response [3]. In our series, patient 5 is currently receiving chemotherapy due to distant metastases, and patient 4 received 30 cycles/60 Gy of radiotherapy due to the presence of residual tumor following resection. Among the 16 previously reported cases of endobronchial LMSs, 13 were alive after a minimum follow-up period of 5 months [3,8]. However, in a larger series of parenchymal LMSs, these tumors have been seen to result in comparatively worse prognosis [16,17].
To conclude, endobronchial SMTs are extremely rare tumors with typically favorable outcomes, depending on degree on differentiation. Accurate diagnosis relies on histopathological examination, for both confirmation of smooth muscle lineage and for determining the malignant potential of the lesion. An appropriate immunohistochemical panel, which includes more than one marker of smooth muscle differentiation, is extremely valuable for differential diagnosis from their morphological mimics, and is necessary for instituting appropriate management.
REFERENCES
1. Dacic S, Elmberger G. Other mesenchymal tumours. In : Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG, editors. WHO classification of tumours of lung, pleura, thymus and heart. Lyon: IARC Press;2015. p. 132–3.
2. Swarnakar R, Sinha S. Endobronchial leiomyoma: a rare and innocent tumour of the bronchial tree. Lung India. 2013; 30:57–60.

3. Elouazzani H, Zouaidia F, Jahid A, Bernoussi Z, Mahassini N. Primary endobronchial leiomyosarcoma of the lung: clinical, gross and microscopic findings of two cases. J Clin Imaging Sci. 2012; 2:35.

4. Cardenas-Garcia J, Lee-Chang A, Chung V, Shim C, Factor S, Tibb A. Bronchial leiomyoma, a case report and review of literature. Respir Med Case Rep. 2014; 12:59–62.
5. Purgina B, Rao UN, Miettinen M, Pantanowitz L. AIDS-related EBV-associated smooth muscle tumors: a review of 64 published cases. Patholog Res Int. 2011; 2011:561548.
6. Awasthi A, Dubey S, Sabhikhi AK, Bal S. Primary endobronchial myxoid leiomyoma in a child: an unusual case report and review of literature. Indian J Pathol Microbiol. 2016; 59:87–9.

7. Litzky L. Epithelial and soft tissue tumors of the tracheobronchial tree. Chest Surg Clin N Am. 2003; 13:1–40.

8. Lee MC, Hsu CP, Hsia JY. Surgical treatment of endobronchial leiomyosarcoma with right main bronchus total obstruction: a case report. Ann Thorac Cardiovasc Surg. 2008; 14:105–8.
9. Kim YK, Kim H, Lee KS, et al. Airway leiomyoma: imaging findings and histopathologic comparisons in 13 patients. AJR Am J Roentgenol. 2007; 189:393–9.

10. Dmello D, Javed A, Espiritu J, Matuschak GM. Endobronchial leiomyoma: case report and literature review. J Bronchology Interv Pulmonol. 2009; 16:49–51.
11. Moran CA, Suster S. Tumors of the lung and pleura. In : Fletcher CD, editor. Diagnostic histopathology of tumors. New York: Churchill Livingstone;2013. p. 207–45.
12. Vaz AP, Magalhães A, Bartosch C, Fernandes G, Marques A. Primary endobronchial leiomyoma: endoscopic laser resection. Rev Port Pneumol. 2011; 17:228–31.
13. Kwon YS, Kim H, Koh WJ, et al. Clinical characteristics and efficacy of bronchoscopic intervention for tracheobronchial leiomyoma. Respirology. 2008; 13:908–12.

14. Harris PF, Maness GM, Ward PH. Leiomyoma of the larynx and trachea: case reports. South Med J. 1967; 60:1223–7.

15. Lee SH, Shim JJ, Shin JS, et al. Primary endobronchial leiomyosarcoma: diagnosis following expectoration of tumor fragment. Respiration. 2001; 68:99–102.
Fig. 1.
Clinical and radiological features in patient 1. (A) Flexible bronchoscopic image showing an endoluminal mass in the right main bronchus. (B) Coronal computed tomography reconstructed image showing a mass lesion in the right main bronchus (case 1).
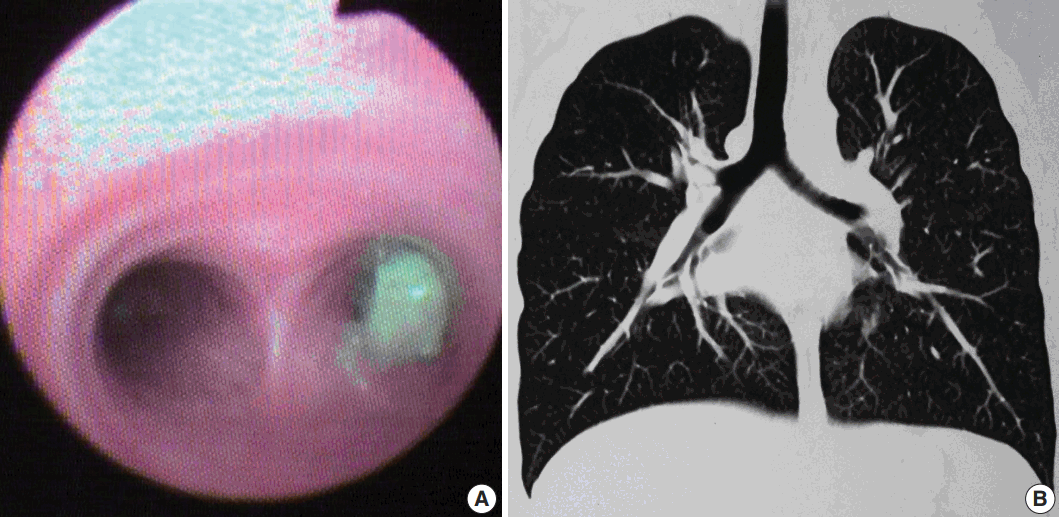
Fig. 2.
Leiomyoma. (A) Photomicrographs of case 3 show bronchial epithelium with a sub-epithelial spindle cell tumor arranged in fascicles. (B) Tumor cells have abundant cytoplasm and ovoid nuclei with homogeneous chromatin. (C, D) Areas of hyalinization and calcification are noted in case 2. Immunohistochemistry shows diffuse smooth muscle actin (E) and desmin positivity (F).
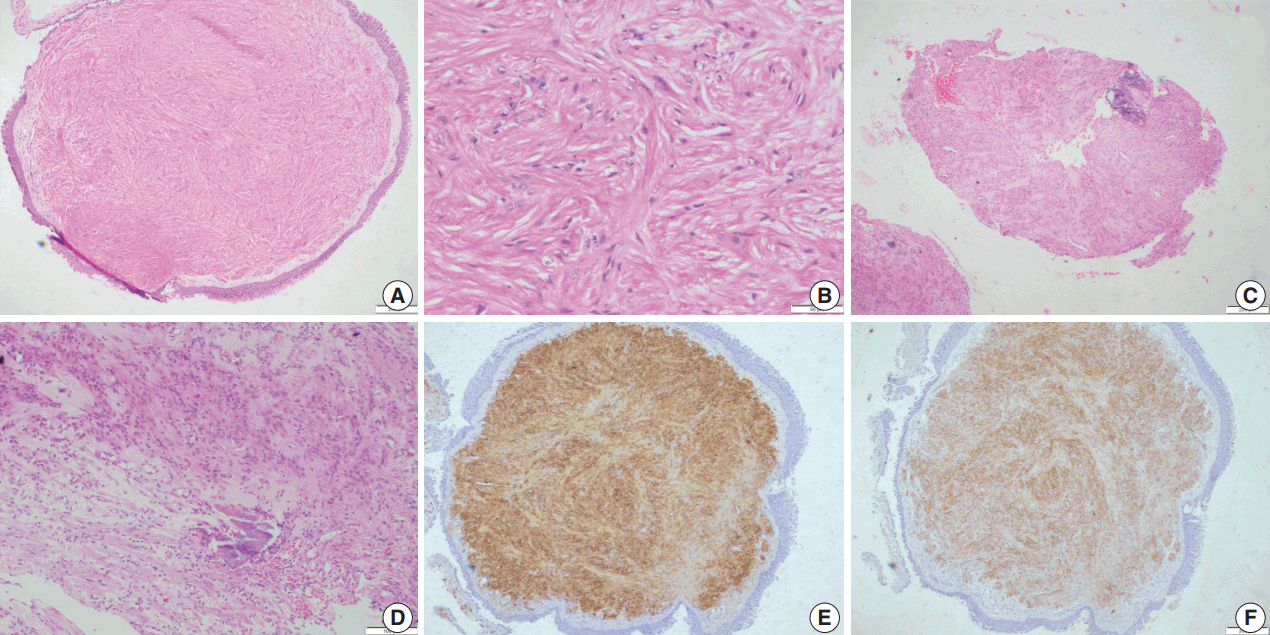
Fig. 3.
Leiomyosarcoma. (A, B)Photomicrographs of the first biopsy from case 4 show small fragments of spindle cell tumor with mild to moderate nuclear pleomorphism and interspersed inflammatory cells; diagnosed as inflammatory myofibroblastic tumor. (C) Excision biopsy showed bronchial epithelium with a cellular spindle cell tumor in the sub-epithelial region. (D) Tumor cells have pale to bright eosinophilic cytoplasm, paranuclear vacuoles (black arrow) with interspersed inflammatory cells and pleomorphic tumor giant cells (red arrow). Immunohistochemistry shows focal positivity for smooth muscle actin (E) and desmin (F).
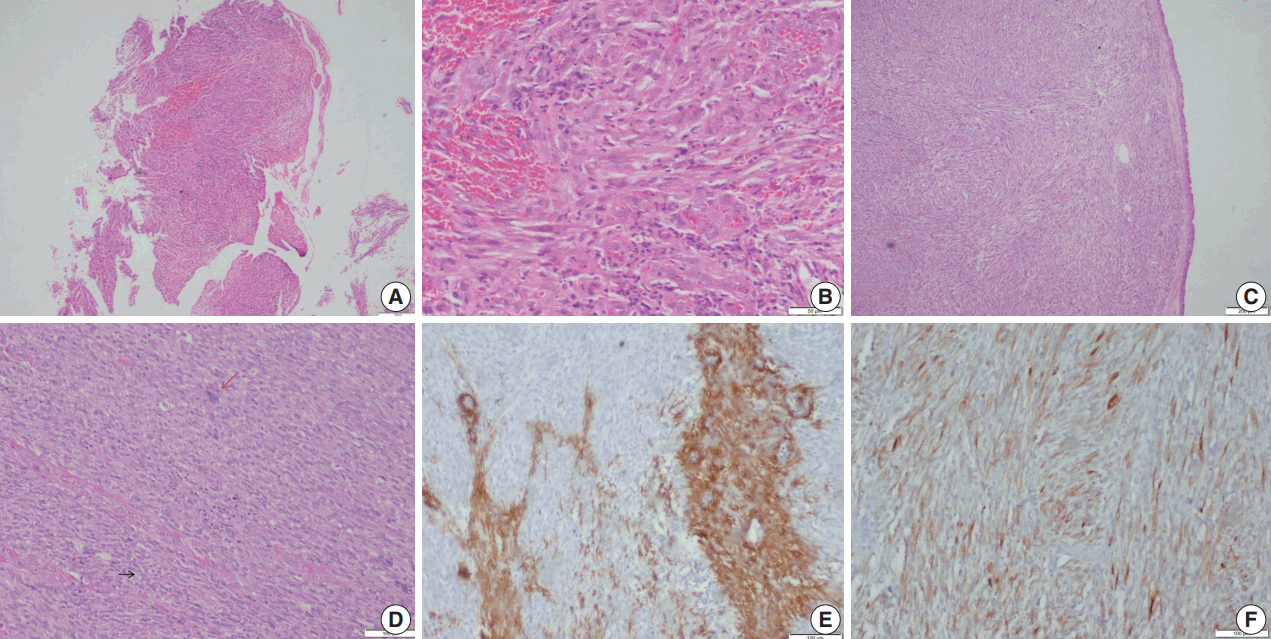
Fig. 4.
Leiomyosarcoma. Photomicrographs of case 5 show multiple fragments of tumor tissue displaying a cellular spindle cell tumor (A) with moderate amounts of eosinophilic cytoplasm, ovoid, hyperchromatic nuclei, paranuclear vacuoles, and mitotic figures (B); perivascular hyalinization is noted (C). (D) Immunohistochemistry shows focal desmin positivity.
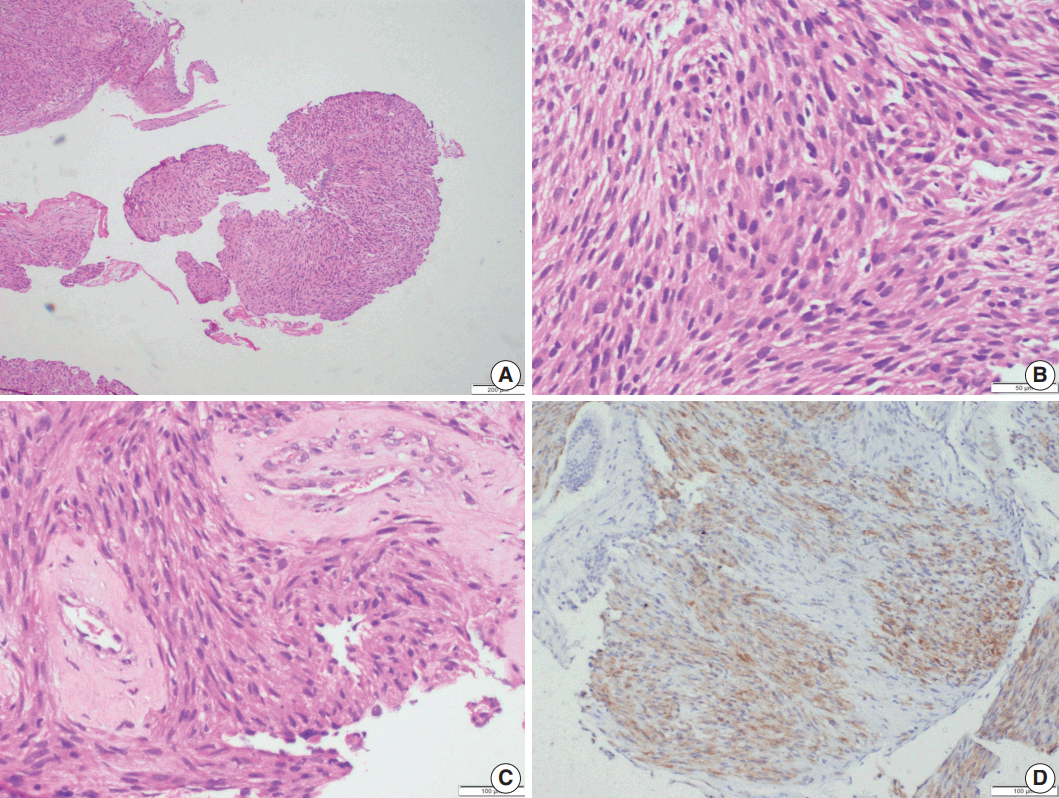
Table 1.
Clinical features, imaging findings and pathological diagnosis of five cases of SMTs
Table 2.
Immunohistochemical features of five cases of endobronchial smooth muscle tumors




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download