In this study, we aimed to evaluate the role of TROP2 expression in PTC based on the fact that TROP2 expression affects the prognosis in diverse epithelial cancers. Knowledge of the role of TROP2 expression, especially in thyroid tumors, has been limited until now. Only three previous studies have evaluated the role of TROP2 expression in thyroid tumors [
12-
14]. These studies mainly focused on the clinical utility of TROP2 expression for differential diagnosis of thyroid tumors, especially in fine-needle aspiration (FNA) specimens. According to these studies, TROP2 expression had a higher sensitivity and specificity for diagnosing PTC than other established markers such as cytokeratin 19, HBME1, and galectin 3, which were commonly used in practice. Therefore, IHC for TROP2 expression was a good diagnostic tool in pathological practices for differential diagnosis of thyroid tumors. In this regard, we wanted to investigate why TROP2 was overexpressed in PTC, but not in other thyroid tumors, and evaluate if the
BRAF mutation was related. To answer this question, we performed IHC to detect TROP2 expression and pyrosequencing to detect
BRAF mutation, and then evaluated the association between TROP2 expression and
BRAF mutation. Consequentially, TROP2 expression was significantly related with
BRAF mutation. We concluded that the diagnostic utility of TROP2 expression came from the
BRAF mutation, which contributes to PTC tumorigenesis. Although there was no mention of an association between
BRAF mutation and TROP2 expression in the abovementioned studies,
BRAF mutation was an important factor influencing TROP2 expression. Therefore, when diagnosing the cases with no TROP2 expression, PTC cannot be ruled out because PTC without
BRAF mutation does occur. In other words, before interpreting TROP2 expression for differential diagnosis of PTC especially with FNA,
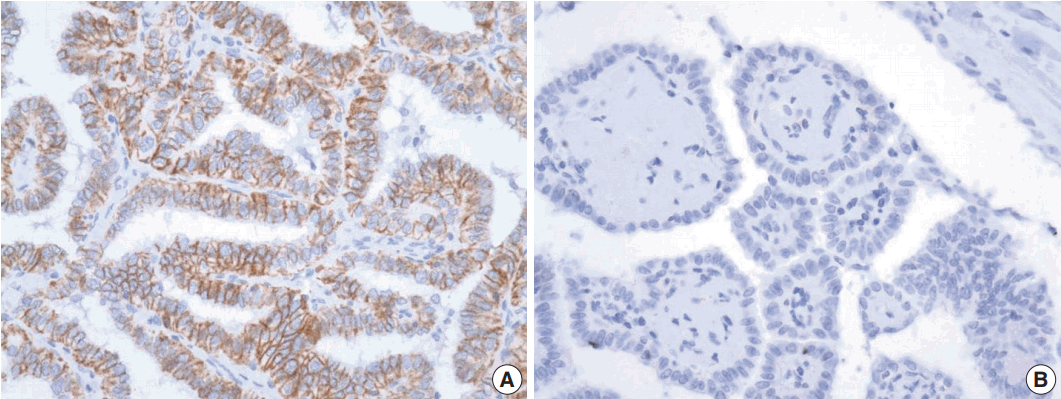
BRAF mutation status should be considered. We also divided PTC into the two histological subtypes, classical and follicular variant of PTC. Most cases of follicular variant of PTC showed no TROP2 immunoreactivity, however, 20 classical PTC cases (47.6%) showed TROP2 immunoreactivity. TROP2 expression had a significant correlation with histological subtype. Therefore, in clinical practice, applying TROP2 IHC may help in the diagnosis of classical PTC cases having
BRAF mutation. We also carried out further
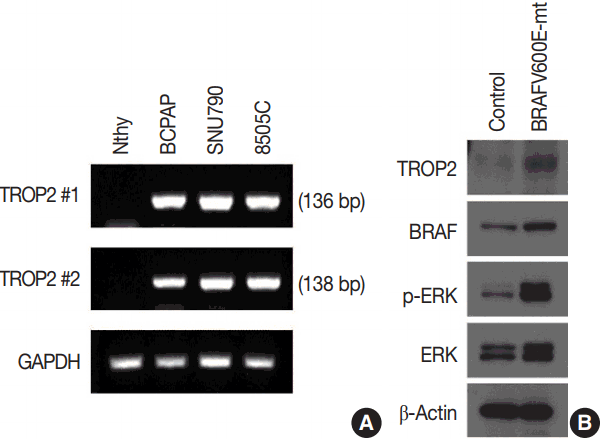
in vitro studies to clarify the association between
BRAF mutation and TROP2 expression. We observed TROP2 mRNA expression in three thyroid cancer cell lines with
BRAF mutation, but no such expression in a normal thyroid cell line. Interestingly, Western blot analysis revealed that TROP2 protein expression was detected in a normal thyroid cell line after introduction of the
BRAF V600E mutation via viral infection. These results support our IHC study of the association between TROP2 expression and the
BRAF V600E mutation in PTC. Expressions of ERK and p-ERK were also increased after introduction of the
BRAF V600E mutation compared to controls. The fact that
BRAF mutations contribute to PTC tumorigenesis by activating the mitogen-activated protein kinase (MAPK) pathway has been well-established [
19,
20]. TROP2 expression also promotes tumorigenesis by activating the MAPK/ERK pathway, which has important implications for various cellular pathways, leading to cancer cell proliferation, migration, invasion, and survival [
21]. Activation of the MAPK/ERK pathway also contributes to tumor invasion and metastasis as well as tumor growth by interacting with other molecules and signaling pathways [
22,
23]. In view of this, we hypothesized that TROP2 expression may be correlated with
BRAF mutation status to directly or indirectly induce ERK pathway activation. ERK pathway activation affects the tumor progression or clinical outcomes. However, our present study did not explain exactly what molecules are involved in activating ERK. Therefore, we will determine the details of signaling between TROP2 and
BRAF mutation in a future study. As previously mentioned, TROP2 overexpression is associated with aggressive biological behavior and poor prognosis in pancreatic, gastric, colorectal, oral, endometrial, and ovarian cancers. However, the relationship between TROP2 expression and PTC outcome remained unclear until now; we assume that TROP2 expression may confer poor PTC prognosis like other epithelial tumors. However, our results did not show a correlation between TROP2 expression and aggressive clinicopathological features including extrathyroidal extension (p = .099) and lymph node metastasis (p = .191). We believe these limitations arose from the relatively small sample size and lack of long-term follow-up. Also, data analysis of the cases divided into two groups according to
BRAF mutation and analysis after combining them into one group should be interpreted with caution. Therefore, further studies with a larger number of cases are required to determine whether TROP2 is an indicator of poor PTC prognosis. In conclusion, we found a significant association between TROP2 expression and
BRAF mutation in PTC. This correlation was confirmed by IHC staining, RT-PCR, and Western blot analysis. Although the molecular players in thyroid cancer progression have not yet been entirely elucidated, TROP2 may be a key molecule to better understand PTC pathogenesis.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download