Abstract
Fine-needle aspiration cytology (FNAC) is a screening test for triaging thyroid nodules, aiding in subsequent clinical management. However, the advantages have been overshadowed by the multiplicity of reporting systems and a wide range of nomenclature used. The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) was formulated in 2007, to give the world a uniform thyroid cytology reporting system, facilitating easy interpretation by the clinicians. Here, we review the status of thyroid FNAC in India in terms of various reporting systems used including a meta-analysis of the previously published data. An extensive literature search was performed using internet search engines. The reports with detailed classification system used in thyroid cytology were included. The meta-analysis of published data was compared with the implied risk of malignancy by TBSRTC. More than 50 studies were retrieved and evaluated. TBSRTC is currently the most widely used reporting system with different studies showing good efficacy and interobserver concordance. Ancillary techniques have, as of now, limited applicability and acceptability in thyroid cytology in India. Twenty-eight published articles met the criteria for inclusion in the meta-analysis. When compared with TBSRTC recommendations, the meta-analysis showed a higher risk of malignancy for categories I and III. Thyroid FNAC is practiced all over India. TBSRTC has found widespread acceptance, with most institutions using this system for routine thyroid cytology reporting. However, reasons for a high malignancy risk for categories I and III need to be looked into. Various possible contributing factors are discussed in the review.
Thyroid cancer is the most common endocrine malignancy, constituting 0.1%–0.2% of all cancers in India with an age-adjusted incidence of 1 per 100,000 in males and 1.8 per 100,000 in females [1]. As per the latest three-year report of 27 population based cancer registries from 2012 to 2014 issued by the National Cancer Registry Program, the incidence was particularly high among females of Papumpare District in Arunachal Pradesh (age adjusted rate 20.7 per 100,000 population), followed by Thiruvananthapuram (13.3) and Kollam Districts (12.0) in Kerala [2].
Thyroid cancers most commonly present as a solitary thyroid nodule. The consensus guidelines from the Endocrine Society of India published a summary of current medical evidence for thyroid nodule management and optimized the guidelines for the clinical practice setting in India [3]. It includes a strong recommendation (Level A) for evaluation of all thyroid nodules > 1 cm, including both palpable and radiologically distinct non-palpable nodules [3]. Prevalence of palpable nodules in India is about 12.2% [4]. India being an endemic area for goiter due to iodine deficiency, it is important to differentiate benign thyroid nodules from malignant ones.
Numerous studies have demonstrated that fine-needle aspiration cytology (FNAC) is a valid procedure for evaluation of thyroid nodules in adults and pediatric population. The role of FNAC is increasing in recent years in management and risk assessment of thyroid nodules.
Cytological evaluation of thyroid swellings is a rapid, easy and inexpensive diagnostic procedure which is widely used as a screening tool. It helps in triaging the patients into candidates for surgical or conservative management. However, the technique has its own shortcomings mainly due to interobserver and intraobserver variability, especially in indeterminate cases. In addition, there is also a lack of uniformity in the reporting systems used, which vary not only from country to country but also from laboratory to laboratory and even among individuals working at the same laboratory. This hampers accurate interpretation by the clinician, thus affecting patient management. To address this common issue, the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) was introduced based upon the proceedings of “The NCI Thyroid Fine Needle Aspiration State of the Science Conference” held in Bethesda, Maryland, in 2007 [5]. TBSRTC encompasses six thyroid cytology categories, with each category having an implied cancer risk and the best modality of management [5].
Here we review the available Indian literature on the status of thyroid aspiration cytology in India along with a meta-analysis of the reviewed data. We performed an extensive literature search in PubMed and Google Scholar databases using the following keywords: “India,” “thyroid,” “cytology,” “cytopathology,” “audit,” “cytology-histology correlation,” “the Bethesda system,” “TBSRTC,” and “FNAC.” Those reports in which a recognizable classification system was used to categorize thyroid cytology smears were included. Case reports and case series were excluded. Cross-references of the selected articles were also checked to look out for additional studies. For meta-analysis, publications with available histopathological correlation were evaluated. The publications which had not used TBSRTC but had used a four or higher-tier system (including “unsatisfactory” category) were reclassified to fit into one of the TBSRTC categories. Hence, “indeterminate cases” were categorized as “atypia of undetermined significance/follicular lesion of undetermined significance” (AUS/FLUS, category III), while “follicular neoplasm,” “follicular patterned lesion” and “Hurthle cell lesion/neoplasm” were classified as “follicular neoplasm/suspicious for a follicular neoplasm” (category IV). Wherever possible, the risk of malignancy (ROM) and the risk of neoplasm (RON) were calculated [6]. Reports with just two categories (benign and neoplastic) besides unsatisfactory and those using histopathological terminologies for imparting cytological diagnoses were not included in the meta-analysis. Papers just providing statistical measures of performance (sensitivity, specificity, negative and positive predictive values) of cytology in comparison with histopathology but not providing details of the histopathological diagnoses were also excluded from the meta-analysis. It should be noted that approximately half of the analyzed articles were published in non-PubMed indexed journals, which may raise an issue regarding quality of the publications. As per our evaluation, studies employed for this review contained sufficient amount of raw or processed data, and hence were eligible for inclusion.
FNAC was first used for cytological diagnosis in 1930s [7]; however, the method has been widely used after 1952 [8]. In India, FNAC has been introduced in early 1970s [9]. First publication on FNAC appeared in 1975 by Gupta et al. [10], which was published in the Indian Journal of Cancer. Needle biopsy of the thyroid had been attempted for the first time in 1965 in India [11], whereas the first paper on FNAC of the thyroid dates back to 1987 by Rege et al. [12] Needle aspiration was initially started without any guidance. Later, with the advent of interventional radiology, the lesional localization was improved. Ultrasound-guided fine-needle aspiration (FNA) is a widely-acclaimed technique in investigating thyroid nodules/lesions. Approximately 50 reports so far have been published based on the Bethesda system and otherwise fulfilling our abovementioned criteria (Table 1) [13-63].
Most studies reclassified cases as per TBSRTC, and compared their distribution data and the ROM in each of the categories (Table 2) [13-63]. Few compared diagnostic accuracy and inter-observer variation of previously used classification systems with TBSRTC. Old classification systems have been used in few of the studies which evaluated sensitivity and specificity of thyroid cytology in accurate diagnoses.
Review of available literature in India and our personal experience suggest that most blind, palpation guided FNAs of thyroid are done by pathologists, whereas clinicians or radiologists perform the FNAC under image guidance and leave the interpretation to pathologists. Although an occasional publication does provide evidence of at least some cases being aspirated by surgical medical officers, by-and-large, palpation-guided thyroid FNA is mostly performed by cytopathologists and ultrasound-guided aspiration by radiologists [60].
In India, palpation-guided FNA appears to be the most commonly used technique, probably being more cost-effective (Table 1). Ultrasound-guided FNA is usually reserved for small or deep-seated poorly palpable nodules. It is also preferable to use ultrasound guidance to aspirate predominantly cystic lesions and for repeat aspiration of a previously non-diagnostic/unsatisfactory aspirate. Only a few centers are using the ultrasound-guided technique for all patients irrespective of the type of the thyroid nodule (Table 1) [34,58].
Interpretation can be done immediately after procedure at the site of FNA or later in the laboratory after staining of aspiration smears. Without rapid on-site evaluation (ROSE), a significant subset of thyroid FNAs are diagnosed inadequate/unsatisfactory for interpretation, which potentially leads to repeat aspirations and additional procedures. The basic purpose of ROSE is to increase the adequacy rate, diagnostic yield, and accuracy of the procedure. Systemic reviews and meta-analysis showed significant reduction in inadequacy rate of thyroid FNAs with ROSE [64,65]. Acquisition of ROSE in routine practice depends upon the infrastructure of the institute which includes availability of manpower, location of the procedure room, case volume, and resources. In our institute, ROSE is offered to all thyroid FNAs under guidance, and it is performed by cytopathologists. Studies on comparison analysis of adequacy assessment of thyroid FNA with and without ROSE were not found in Indian literature. We believe ROSE is practiced only in a few academic institutions in India.
The Indian Academy of Cytologists (http://www.cytoindia.com) conducts examination for cytotechnicians and cytotechnologists. There are few centers which run cytotechnician and cytotechnologist training programs for certification. Cytotechnologists work as cytoscreeners; however, in India only limited institutions have cytoscreeners. Their work allocation depends upon the institutional work requirement and administration policies.
The External Quality Assurance Programme of the Indian Academy of Cytologists is aimed to maintain and monitor the quality of reporting on all cytopathology specimens, in which over 100 cytopathology laboratories from all over the India participate for FNA, exfoliative specimens and cervical smears. In terms of thyroid FNA, only straightforward diagnoses (such as lymphocytic thyroiditis or carcinomas) are assessed and so TBSRTC is not strictly followed, unlike cervical smears where it is mandatory to diagnose lesions according to the Bethesda classification (personal communication with Prof. Radhika Srinivasan, Postgraduate Institute of Medical Education and Research, Chandigarh, India).
The needles used in thyroid FNA vary in size from 21G to 28G, with or without aspiration for fine needle aspiration cytology and fine needle capillary sampling, respectively (Table 1). The most commonly used needle was 23G followed by 24G in published studies. Since the thyroid is a highly vascular organ, and given the risk of hemorrhagic complications, it is advisable to use a small-bore needle (25–27 gauge). The unstained smear may then be visually evaluated for tissue fragments and/or colloid, and if required, a larger bore needle may be used for subsequent aspirations [5,66]. Larger diameter needles are also preferable for draining thick colloid [5].
Most institutes use direct smears in which the material is smeared onto the glass slide and either kept air-dried for Romanowsky stains (May-Grünwald-Giemsa, Leishman, Giemsa) stain or wet-fixed with common fixatives (95% ethyl alcohol, 95% methanol, 95% isopropyl alcohol, or a solution of ether and 95% alcohol) for Papanicolaou and/or hematoxylin and eosin stain (H&E). Papanicolaou and H&E stains help in characterization of nuclear features whereas Romanowsky stains better define cytoplasmic characteristics. In case of cystic nodules, aspirated fluid is centrifuged and smears are prepared from the sediment. Most cytopathologists in India use a combination of Romanowsky and Papanicolaou stains. However, H&E is preferred in a few institutions due to its cost effectiveness and better familiarity of the stain from surgical pathology.
Liquid-based cytology (LBC) is another adjunctive technique in thyroid FNA which is associated with better preservation of cellular details. It removes obscuring hemorrhage, cellular debris and inflammatory cells to a large extent from the background. Keyhani et al. [67] have compared conventional, cell block and LBC preparations in a cohort of 100 patients with thyroid nodules. While a significant percentage (87%) of cases yielded informative results using LBC method, only 69% of the samples processed for cell blocks were informative. Both techniques had almost equal sensitivity (95% for LBC vs 96% for cell block), but the specificity of LBC (31%) was reported to be higher than that of the cell block (24%). It has been suggested that LBC may be used as a supplementary technique to conventional smears to improve the diagnostic yield of thyroid aspiration cytology. In a more recent study by Prasad et al. [68], LBC slides from 41 cases of thyroid swellings (23 nodular colloid goiter, 14 thyroiditis, and 4 carcinoma) were assessed and compared with conventional smears. Importantly, the authors cautioned against the regular use of LBC in thyroid cytology. While the amount of background colloid was reported to be significantly diminished, they found nuclear features (grooves and pseudoinclusions) of papillary thyroid carcinoma less forthcoming on LBC. Another study evaluated 18 cases of thyroid swellings (10 colloid goiter, four thyroiditis, and four carcinoma cases) by LBC and compared the results with conventional smears [69]. While the technique was not found to be of much use in benign thyroid diseases, it was beneficial in diagnosis of neoplastic lesions. However, the small number of cases evaluated precludes any definite interpretation. To conclude, data reported from Indian institutions suggest that LBC may be used as an adjunct method but cannot replace conventional smears in thyroid cytology.
Reporting of thyroid aspirate smears has evolved tremendously over the past decade. Studies from pre-Bethesda era showed usage of a range of formats for reporting. These include descriptive reporting, use of histopathology equivalents, and variably tiered classification systems, ranging from just two categories (non-neoplastic and neoplastic) to four or five categories (Table 1). In the past and even today in some centers across the country, a range of formats are being used. Histopathology correlates when used are easily interpretable by the clinicians, however, they may not be perfectly applicable to all thyroid aspirates, especially the gray zone lesions: for example, follicular neoplasms, hyperplastic thyroid nodules versus follicular adenoma, papillary hyperplasia versus papillary thyroid carcinoma, or reactive change versus papillary thyroid carcinoma. These cytodiagnostic categories do not provide management guidelines to clinicians. The 2-tier system suffers from similar shortcomings, and can lead to over- as well as under-treatment. A 3- or 4-tiered system has a drawback of inadvertent clubbing of benign and malignant cases. Benign lesions such as hyperplastic nodules and Hashimoto’s thyroiditis with nuclear atypia are combined together with follicular/Hurthle cell neoplasms, non-invasive follicular thyroid neoplasm with papillary like nuclear features (NIFTP) and carcinomas with poor preservation or less cellularity.
After introduction of the 6-tiered TBSRTC [5], several cytopathologists tested its efficacy and reported the ROM in different categories. Pathak et al. [29] reclassified over 400 thyroid aspirates as per TBSRTC and found strong agreement level among the three observers (Fleiss’ kappa score, 50.7) and a significant reduction in the number of inconclusive diagnoses (p < .001) while using TBSRTC. In another study, TBSRTC was also found to be superior in terms of sensitivity (100% vs 77%) and specificity (82.5% vs 69%) in comparison with the conventional system [42]. Upon comparing three thyroid cytology reporting systems, which included conventional (unsatisfactory, benign or negative for malignancy, follicular lesions, indeterminate, positive for malignancy), the British Thyroid Association/the Royal College of Pathologists (BTA/RCP) (Thy 1–Thy 5 categories) and TBSRTC, TBSRTC and BTA/RCP were found to be better in terms of approachability, classification of thyroid lesions, treatment and follow-up than the conventional reporting system [70].
In cytology, every FNA from any organ system must be evaluated for adequacy in the proper context of clinical and radiological findings. To decrease the false-negative rate, TBSRTC has laid down criteria for adequacy. For accurate interpretation, TBSRTC recommended any thyroid FNA specimen to be considered satisfactory for evaluation when at least six groups of well-preserved, well-stained, and well-visualized follicular cells are seen on the aspirate and each group is composed of at least 10 follicular epithelial cells, preferably on a single slide [5]. There are certain exceptions to this rule, such as solid nodules with cytologic atypia, solid nodules with inflammation and colloid nodules. Cyst fluid with less than six groups is considered non-diagnostic/unsatisfactory unless clinical and radiological features are suggestive of a benign cyst [5].
Pre-Bethesda era studies considered an FNA as unsatisfactory/non-diagnostic when there was less cellularity (no objective quantification) and when excessive blood or poor technical quality obscured smears such as overtly thick smears and air drying of alcohol-fixed smears. Now, most cytopathologists use objective adequacy criteria laid down by TBSRTC, except for a few who use the Royal College guidelines (Table 1) [36].
Since inception of TBSRTC in 2008, most laboratories in India have adopted it. However, a few studies have used a different set of criteria [47]. While TBSRTC requires 5–6 groups of well-preserved follicular epithelial cells with 10 or more cells per group, Tagore et al. [47] claimed that in case of large clusters of follicular epithelial cells, 10 clusters were needed with each having more than 20 cells. In case of presence of tissue fragments, the minimum number of fragments required was 8 [47]. The Royal College of Pathologists (RCP) guidelines were used in one of the studies, which were similar to TBSRTC in terms of cellularity and approach to cystic lesions, i.e., minimum of six groups of follicular cells across all the submitted slides, each with at least 10 well-visualized epithelial cells [36]. Samples containing mostly macrophages but lacking enough cells and/or abundant colloid were also considered “unsatisfactory,” similar to cases with cellular details obscured by blood/clotting or crushing artifact/poor fixation/poorly spread smears. However, there were others who have just mentioned “blood only/lack of cellularity/poor quality smears/presence of obscuring factors” as the reason for calling a sample unsatisfactory [15,22,24]. Still, some studies have not specified the criteria used for adequacy [27,28,57].
Of the various ancillary techniques which can be utilized in cytology as diagnostic aid, cell block, immunocytochemistry (ICC) and flow cytometry are probably the most commonly used. Ancillary studies in thyroid cytology are more useful in rare borderline cases of medullary carcinoma [71], anaplastic carcinoma [72], metastases and lymphomas [73], than in the more common papillary thyroid carcinoma. ICC could be used for diagnosing tuberculous thyroiditis, in which Ziehl-Neelsen staining had failed to reveal acid-fast bacilli [74]. ICC is a cost-effective and easy technique which can be performed on alcohol or acetone-fixed unstained as well as destained cytology smears and better, if available, on cell blocks [75-79]. Nevertheless, its role in differentiating benign from malignant thyroid nodules of follicular cell origin is limited with contradictory results in different studies [75].
Cell block is a complementary method of assessing cytology material, which gained importance because of the advantages it has over conventional cytology smears. Cell block is similar to a mini-biopsy, since it imparts better-preserved tissue architecture and provides several sections, which can be utilized to perform a battery of ancillary tests including special stains, immunohistochemistry, ultrastructural studies, and molecular tests [80]. Although few studies have shown utility of cell blocks as an adjunct to conventional cytology in diagnosis of thyroid tumors [81], their use in everyday clinical practice is limited by their low cellularity, enhanced cost and turnover time [80]. As per the available literature, the technique is being done in routine only in rare centers across India [44].
Molecular testing for BRAF mutation and other molecular alterations, as per American Thyroid Association 2015 guidelines, may be used to supplement malignancy risk assessment, especially in indeterminate cytology [82]. However, there is not much published data on the utility of thyroid FNA molecular testing in India. Despite a thorough search, we could find only one abstract, whereby the authors had retrospectively evaluated 40 thyroid aspirate samples for BRAF mutation by Sanger sequencing, with effective amplification achieved in over half of them [83]. The mutation was detected in 12% of papillary thyroid carcinomas, which was significantly lower than the expected rate [83,84]. Rare reports of fluorescence in-situ hybridization on cytology smears of thyroid are also available [73].
While FNAC is the most common primary diagnostic modality for diagnosing follicular-derived thyroid tumors, cases with clinical, cytological or radiological features suggestive of non-follicular cell derived thyroid malignancy are subjected to tru-cut or open biopsy, as it gives the additional advantage of architectural preservation and performing immunohistochemistry. It is of use especially for hematolymphoid neoplasms [85-88].
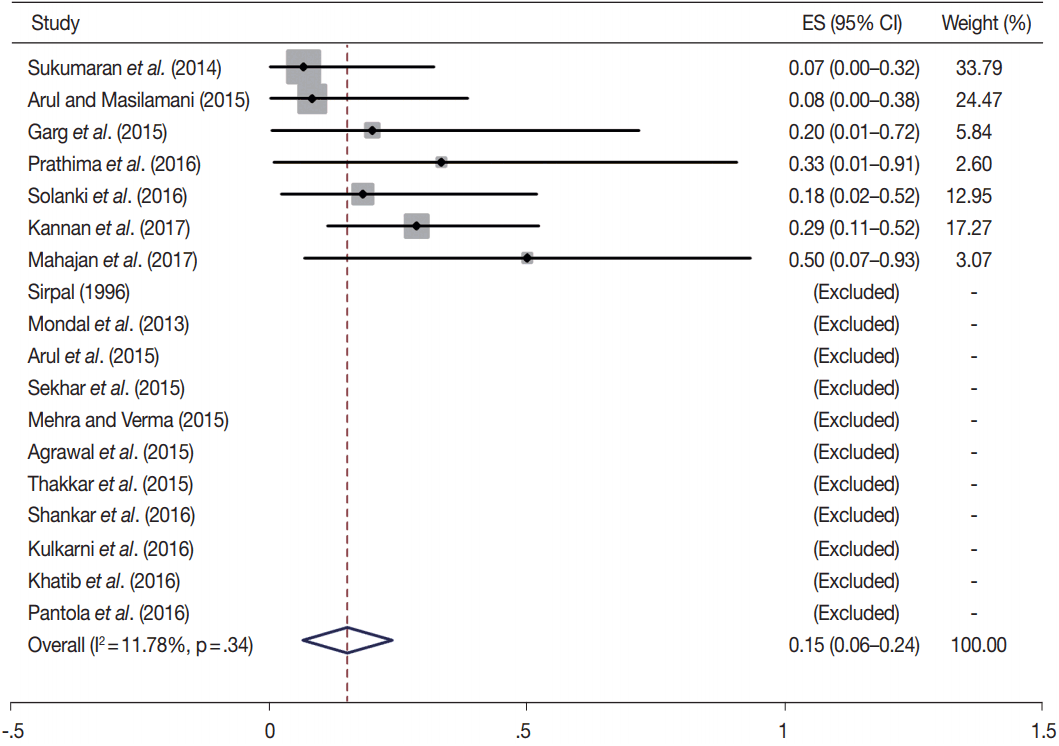
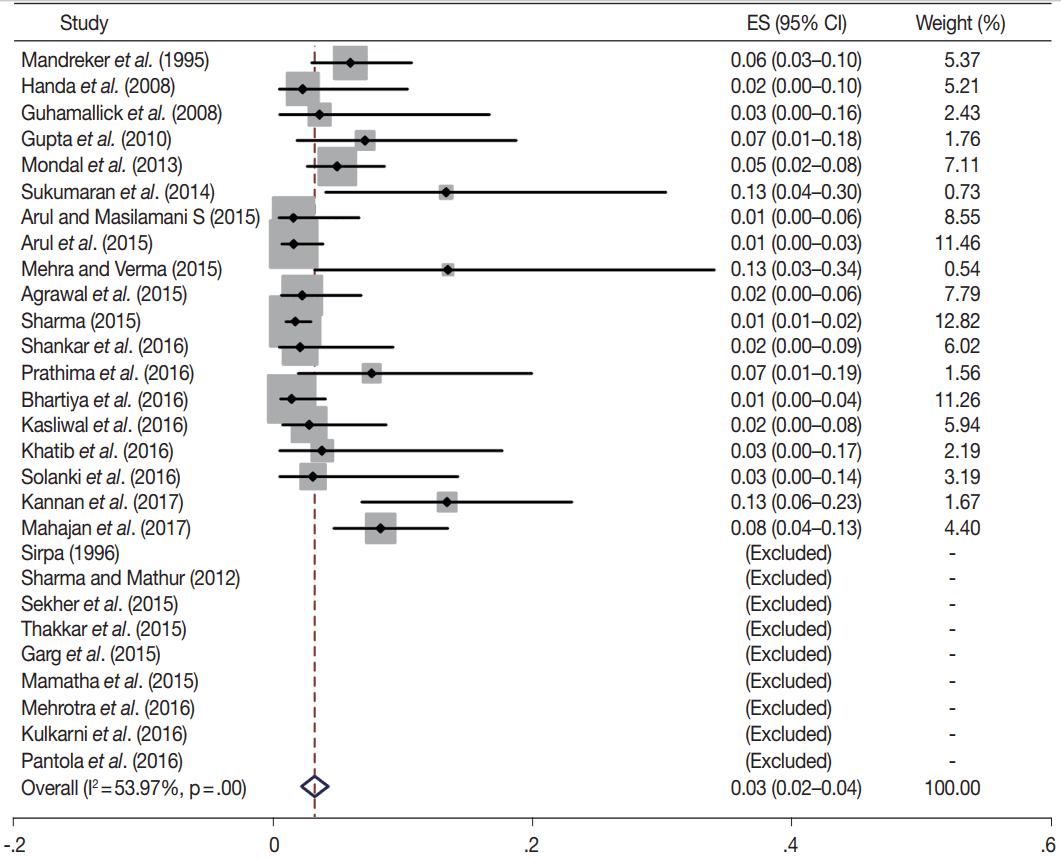
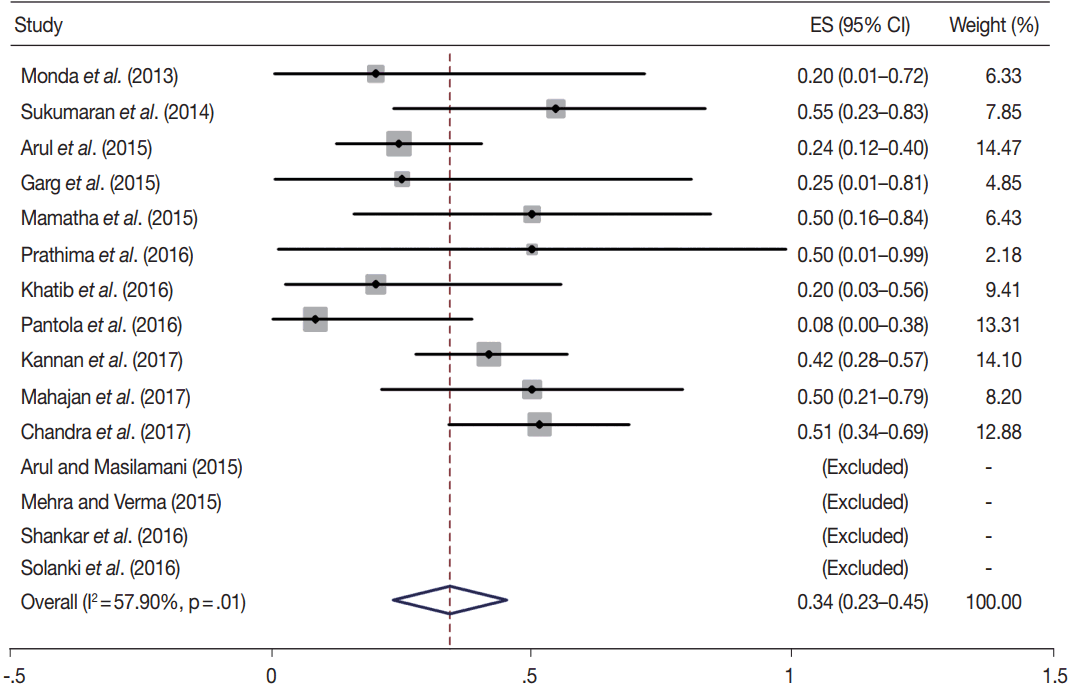
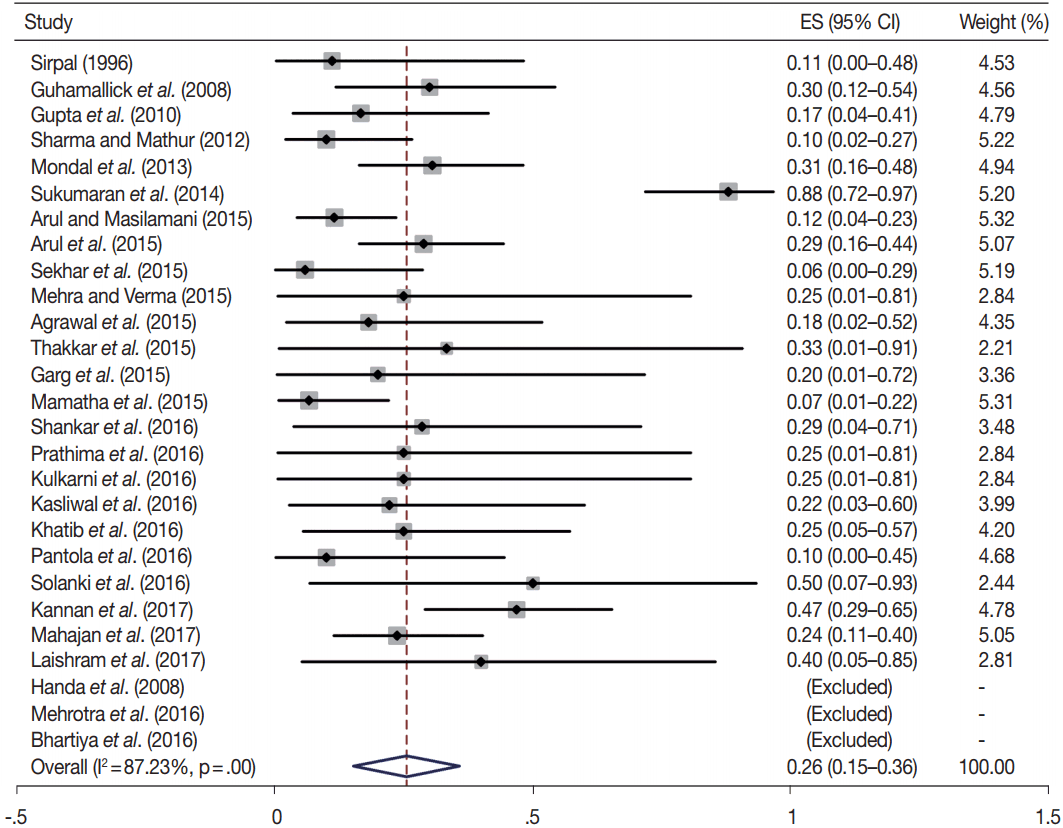
Owing to the variable number of cases included in various studies, in order to get an accurate overall assessment of the ROM in different categories, a metaanalysis was performed. Of the 52 articles selected for the review, 28 met the inclusion criteria for meta-analysis (Table 3). It was done using STATA ver. 12.0 (Stata Corp, College Station, TX). Random effects model was used to calculate the pooled estimate. A p < .05 was considered statistically significant.
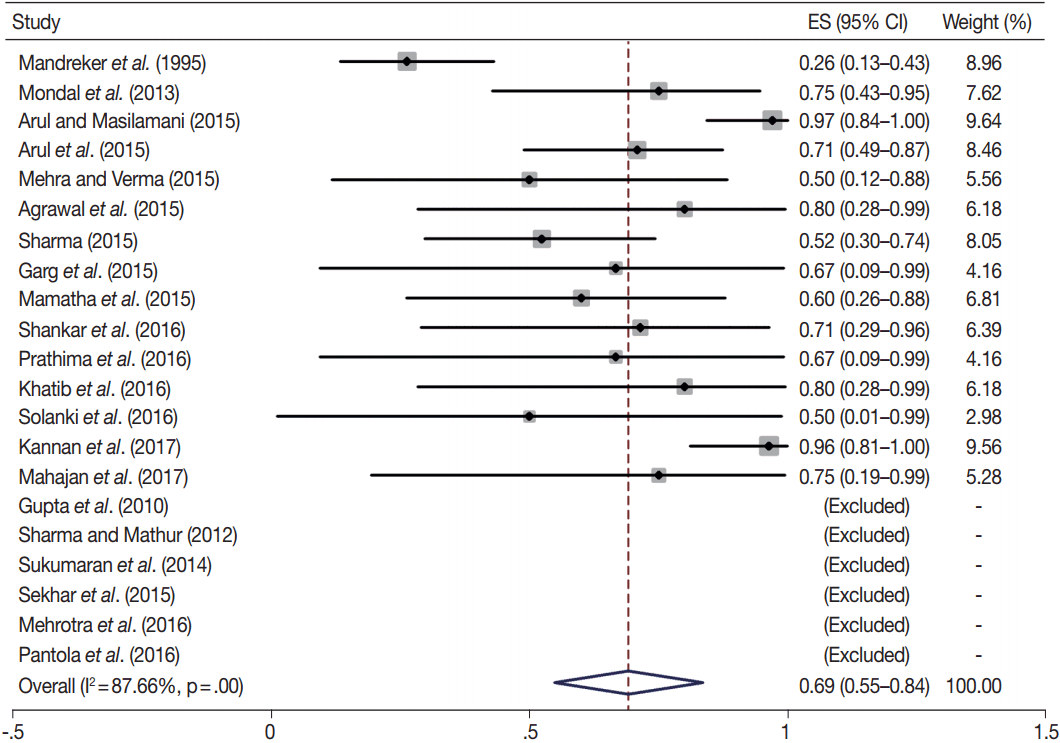
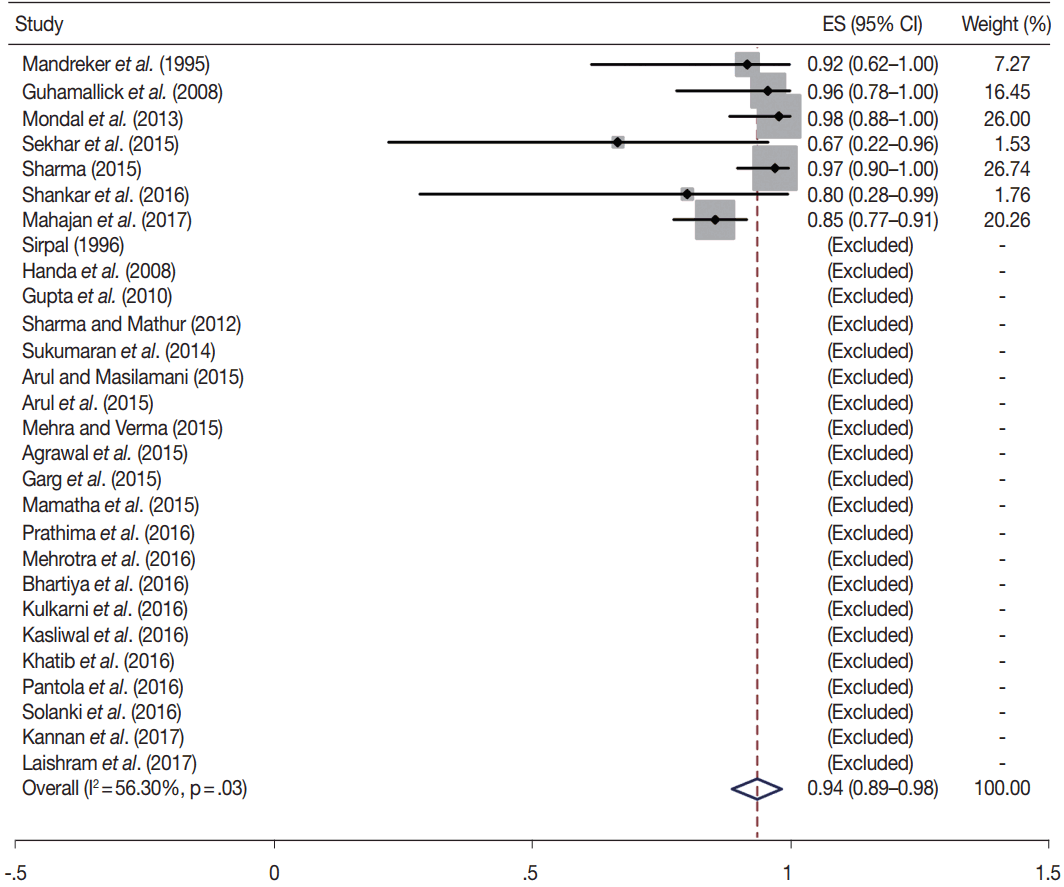
TBSRTC has ascribed a particular ROM to every Bethesda category (Table 3). Meta-analysis revealed a higher ROM for the category III as compared to TBSRTC estimates, 34% versus 5%– 15% (Figs. 1–6). Recent meta-analyses by Straccia et al. [89] and Krauss et al. [6] also found a high ROM (27% and 24%, respectively) for category III. Interestingly, we have also found a ROM of 34.3% (unpublished data) at our institute. Category III includes cases which are neither undoubtedly benign nor can be categorized into higher categories of IV and higher. It is so heterogeneous that sub-classification of AUS/FLUS has been recommended by some authors, based on the presence of architectural and/or nuclear atypia, improving cancer risk estimation [90-93]. Although TBSRTC defines AUS/FLUS category as last resource category and should only be ≤ 7% of total thyroid FNAs, it was found out to be a heterogeneous category which ranged from < 1% to > 5% in different studies (Table 2). We have an approximately 11% rate of AUS/FLUS category in our institute (unpublished data).
Cases with poor cellularity and/or technical quality having some atypia are also huddled into this category. Our institute is a teaching hospital, where aspiration is performed by residents. Hence, lack of adequate experience of the aspirator results in hemodilution and poor smear preparation and may contribute to a higher ROM of category III. Re-aspirating such cases along with radiological correlation, to some extent, decreases the proportion of cases in this category as well as the ROM. Category I also had a higher ROM and may be explained by the same reason.
Impact of the recent reclassification of non-invasive encapsulated follicular variant of papillary carcinoma of thyroid into NIFTP is not evident in this review as most studies included are from pre-NIFTP era. Studies post-NIFTP introduction have not specified it in their histopathological diagnoses. It is likely that the impact, particularly a decrease of ROM for the indeterminate diagnostic categories [94], is dependent on the incidence of NIFTP, which is relatively low in our settings (unpublished data), consistent with Asian data [95].
In addition, a wide 95% confidence interval (CI) was noted for categories III (23%–45%), IV (15%–36%), and V (55%–84%). The wide range may be attributed to interobserver variation and differences in experience levels of the pathologists. As the extreme entities (benign and malignant) are the easiest to categorize in a well-prepared aspirate, the CI values in the two categories were low. Krauss et al. [6] in their recent meta-analysis also reported a wide CI for categories III, IV, and V. The authors ascribed it to subjective differences in the interpretation of the Bethesda criteria for diagnosis of these categories, and recommended introduction of a performance measure such as ratio of AUS/FLUS to total thyroid FNAs for each laboratory to follow. As expected, RON which includes benign tumors (the most common being follicular adenoma) was higher than the ROM (Table 3).
Thyroid FNAC is practiced all over India in academic and private institutes as well as private hospitals and laboratories. In India, most thyroid aspiration samples are collected by pathologists, using manual palpation. Most centers prepare both alcohol-fixed and air-dried smears stained with Papanicolaou/H&E and May-Grünwald-Giemsa, respectively. TBSRTC is currently the most widely used reporting system with different studies showing good efficacy and interobserver concordance. Ancillary studies including core biopsy and molecular testing, as of now, have limited applicability and acceptability in thyroid cytology in India. Category III is the most heterogeneous category with a wide range of ROM and RON. Case to case discussion among the clinicians and pathologists supplemented by radiological correlation may help improve the management of these patients.
ACKNOWLEDGMENTS
We sincerely thank Dr. Andrey Bychkov, MD, PhD, Department of Pathology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand, for his expert guidance and help in manuscript editing.
REFERENCES
1. Gangadharan P, Nair MK, Pradeep VM. Thyroid cancer in Kerala. In : Shah AH, Samuel AM, Rao RS, editors. Thyroid cancer: an Indian perspective. Mumbai: Quest Publications;1999. p. 17–32.
2. National Centre for Disease Informatics and Research. National Cancer Registry Programme Indian Council of Medical Research. Three-year report of population based cancer registries 2012-2014. Bengaluru: NCDIR-NCRP;2016.
3. Unnikrishnan AG, Kalra S, Baruah M, et al. Endocrine Society of India management guidelines for patients with thyroid nodules: a position statement. Indian J Endocrinol Metab. 2011; 15:2–8.

4. Usha Menon V, Sundaram KR, Unnikrishnan AG, Jayakumar RV, Nair V, Kumar H. High prevalence of undetected thyroid disorders in an iodine sufficient adult south Indian population. J Indian Med Assoc. 2009; 107:72–7.
5. Baloch ZW, LiVolsi VA, Asa SL, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008; 36:425–37.

6. Krauss EA, Mahon M, Fede JM, Zhang L. Application of the Bethesda classification for thyroid fine-needle aspiration: institutional experience and meta-analysis. Arch Pathol Lab Med. 2016; 140:1121–31.

8. Soderstrom N. Puncture of goiters for aspiration biopsy. Acta Med Scand. 1952; 144:237–44.
9. Das DK. Fine-needle aspiration cytology: its origin, development, and present status with special reference to a developing country, India. Diagn Cytopathol. 2003; 28:345–51.

10. Gupta SK, Dutta TK, Aikat M, Gupta BD, Talwar BL, Aikat BK. Evaluation of fine needle aspiration biopsy technique in the diagnosis of tumours. Indian J Cancer. 1975; 12:257–67.
12. Rege JD, Nath AR, Bijlani JC, Trivedi DR, Deshpande DV. Fine needle aspiration cytology in solitary cold nodules of thyroid. J Assoc Physicians India. 1987; 35:819–21.
13. Mandreker SR, Nadkarni NS, Pinto RG, Menezes S. Role of fine needle aspiration cytology as the initial modality in the investigation of thyroid lesions. Acta Cytol. 1995; 39:898–904.
14. Sirpal YM. Efficacy of fine needle aspiration cytology in the management of thyroid diseases. Indian J Pathol Microbiol. 1996; 39:173–8.
15. Handa U, Garg S, Mohan H, Nagarkar N. Role of fine needle aspiration cytology in diagnosis and management of thyroid lesions: a study on 434 patients. J Cytol. 2008; 25:13–7.

16. Guhamallick M, Sengupta S, Bhattacharya NK, et al. Cytodiagnosis of thyroid lesions: usefulness and pitfalls: a study of 288 cases. J Cytol. 2008; 25:6–9.
17. Gupta M, Gupta S, Gupta VB. Correlation of fine needle aspiration cytology with histopathology in the diagnosis of solitary thyroid nodule. J Thyroid Res. 2010; 2010:379051.

18. Bagga PK, Mahajan NC. Fine needle aspiration cytology of thyroid swellings: how useful and accurate is it? Indian J Cancer. 2010; 47:437–42.

19. Sengupta A, Pal R, Kar S, Zaman FA, Sengupta S, Pal S. Fine needle aspiration cytology as the primary diagnostic tool in thyroid enlargement. J Nat Sci Biol Med. 2011; 2:113–8.
20. Renuka IV, Saila Bala G, Aparna C, Kumari R, Sumalatha K. The Bethesda System for Reporting Thyroid Cytopathology: interpretation and guidelines in surgical treatment. Indian J Otolaryngol Head Neck Surg. 2012; 64:305–11.

21. Sharma R, Mathur DR. Diagnostic accuracy of fine needle aspiration cytology (FNAC) of the thyroid gland lesions. Int J Health Sci Res. 2012; 2:1–7.
22. Patel MM, Patel K, Kaptan KR, Italiya SL, Saini G. Fine needle aspiration cytology as a first line investigation in thyroid lesions. Natl J Med Res. 2013; 3:106–10.
23. Mondal SK, Sinha S, Basak B, Roy DN, Sinha SK. The Bethesda system for reporting thyroid fine needle aspirates: a cytologic study with histologic follow-up. J Cytol. 2013; 30:94–9.

24. Kukar N, Malhotra V, Saluja M. Analysis of fine needle aspiration cytology of thyroid lesions. Internet J Pathol. 2013; 15:1–9.
25. Bhasin TS, Mannan R, Manjari M, et al. Reproducibility of ‘The Bethesda System for Reporting Thyroid Cytopathology’: a multicenter study with review of the literature. J Clin Diagn Res. 2013; 7:1051–4.

26. Borgohain R, Lal RK, Chatterjee P, Brahma N, Khanna S. A study of cyto-histological correlation in the diagnosis of thyroid swelling. IOSR J Dent Med Sci. 2014; 13:46–9.

27. Mangshetty SS, Jewargikar R, Andola SK. Fine needle aspiration cytology of 220 thyroid lesions with histopathological correlation. Int J Res Health Sci. 2014; 2:243–53.
28. Panchal MG, Deshpande SA, Noone RB, Suvernakar SV, Meshram DP. Diagnostic accuracy fine needle aspiration cytology of thyroid gland lesions. Int J Pharm Sci Invent. 2014; 3:5–10.
29. Pathak P, Srivastava R, Singh N, Arora VK, Bhatia A. Implementation of the Bethesda System for Reporting Thyroid Cytopathology: interobserver concordance and reclassification of previously inconclusive aspirates. Diagn Cytopathol. 2014; 42:944–9.

30. Sukumaran R, Kattoor J, Pillai KR, et al. Fine needle aspiration cytology of thyroid lesions and its correlation with histopathology in a series of 248 patients. Indian J Surg Oncol. 2014; 5:237–41.

31. Arul P, Masilamani S. A correlative study of solitary thyroid nodules using the Bethesda System for Reporting Thyroid Cytopathology. J Cancer Res Ther. 2015; 11:617–22.

32. Arul P, Akshatha C, Masilamani S. A study of malignancy rates in different diagnostic categories of the Bethesda System for Reporting Thyroid Cytopathology: an institutional experience. Biomed J. 2015; 38:517–22.

33. Sekhar A, Inamdae SS, Dombale VD, Prabhu MH. Fine needle aspiration cytology study of thyroid lesions: a 2 year prospective study in a tertiary centre. Int J Pharm Biol Sci Arch. 2015; 3:15–9.
34. Mehra P, Verma AK. Thyroid cytopathology reporting by the Bethesda system: a two-year prospective study in an academic institution. Patholog Res Int. 2015; 2015:240505.

35. Agrawal R, Saxena M, Kumar P. A study of fine needle aspiration cytology of thyroid lesions with histopathological correlation. Indian J Pathol Oncol. 2015; 2:277–83.

36. Sharma C. Diagnostic accuracy of fine needle aspiration cytology of thyroid and evaluation of discordant cases. J Egypt Natl Canc Inst. 2015; 27:147–53.

37. Thakkar B, Patel PJ, Mangar U, Shiladariya P, Suthar NB, Patel P. Retrospective study of fine needle aspiration cytology of thyroid lesions according to the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC). Int J Res Med. 2015; 4:132–6.
38. Garg S, Desai NJ, Mehta D, Vaishnav M. To establish Bethesda system for diagnosis of thyroid nodules on the basis of FNAC with histopathological correlation. J Clin Diagn Res. 2015; 9:EC17–21.
39. Kathirvel C. Criteria for cytological diagnosis of thyroid lesions. Otolaryngol Online J. 2015; 5(Suppl 2):10–9.
40. Alagarsamy J, Sivaraman R, Kadhirvel V. Accuracy of fine needle aspiration cytology and incidence of benign/malignant tumours in solitary thyroid nodules. Int J Allied Med Sci Clin Res. 2015; 3:193–9.
41. Hathila R, Patel S, Vaghela P, Makwana G, Parmar P. Cytology findings of the thyroid lesions with the histopathology findings correlation. Int J Med Sci Public Health. 2016; 5:642–6.

42. Mamatha M, Sekhar SC, Rani HS, Anil SS, Vandana G. A comparative study between conventional system and the Bethesda system applied for reporting thyroid cytopathology. Int Arch Integr Med. 2015; 2:87–95.
43. Gupta V, Bhake A, Dayal S. Pattern and frequency of thyroid pathologies among thyroid cytology specimen in rural part of central India: a retrospective secondary data analysis. Thyroid Res Pract. 2015; 12:93–5.

44. Shankar SP, Meenakshisundaram K, Rajalakshmi V, Selvakumar SA, Giridharan B. The Bethesda System for Reporting Thyroid Cytopathology: a two year retrospective review in a tertiary care hospital. Indian J Pathol Oncol. 2016; 3:48–54.

45. Prathima S, Suresh TN, Harendra Kumar ML, Bhaskaran A. Impact of the Bethesda system in reporting thyroid cytopathology. Thyroid Res Pract. 2016; 13:9–14.

46. Mehrotra D, Anita AM, Andola SK, Patil AG. Thyroid cytology evaluation based on the Bethesda system with clinico-morphological correlation. Ann Pathol Lab Med. 2016; 3:A347–355.
47. Tagore S, Jayaprakash HT, Sasidharan A, Nagaraj T, Santosh HN, Balraj L. Cytological study of thyroid lesions by fine-needle aspiration cytology. J Med Radiol Pathol Surg. 2016; 2:12–5.

48. Kalita DJ, Das B. A three years study of FNAC of thyroid lesions in a tertiary care hospital. Indian J Appl Res. 2016; 6:559–61.
49. Bhartiya R, Mallik M, Kumari N, Prasad BN. Evaluation of thyroid lesions by fine-needle aspiration cytology based on Bethesda System for Reporting Thyroid Cytopathology classification among the population of South Bihar. Indian J Med Paediatr Oncol. 2016; 37:265–70.

50. Kulkarni CV, Mittal M, Nema M, Verma R. Diagnostic role of the Bethesda System for Reporting Thyroid Cytopathology in an academic institute of Central India: one year experience. Indian J Basic Appl Med Res. 2016; 5:157–66.
51. Lohiya V, Kalla AR, Bharadwaj V. Utilisation of Bethesda System for Reporting Thyroid Cytopathology: a study of 250 cases. Int J Appl Res. 2016; 2:103–7.
52. Kasliwal N, Tanwar S, Pachori G, Gupta N, Maheshwari N, Jain D. Usefulness of preoperative FNAC of thyroid swelling along with application of Bethesda system of reporting. Int J Med Res Prof. 2016; 2:204–9.

53. Khatib Y, Mulla A, Patel RD, Momin E, Gite V, Khade A. Classification of thyroid FNA smears into Bethesda categories and their correlation with thyroid function tests. Sch J Appl Med Sci. 2016; 4:916–23.
54. Pantola C, Kala S, Khan L, Pantola S, Singh M, Verma S. Cytological diagnosis of pediatric thyroid nodule in perspective of the Bethesda System for Reporting Thyroid Cytopathology. J Cytol. 2016; 33:220–3.

55. Babu SB, Raju R, Radhakrishnan S. Correlation of fine needle aspiration cytology with histopathology in the diagnosis of thyroid swellings. Int Surg J. 2016; 3:1437–41.

56. Solanki R, Chaudhary VK, Sharma M, Ansari M. Validity assessment of ‘the Bethesda System for Reporting Thyroid Cytopathology’. Int J Health Sci Res. 2016; 6:126–32.
57. Aramani SS, Gururajaprasad C. A cytohistopathological correlation of thyroid lesions with critical evaluation of discordant cases: an experience at a tertiary care hospital. Ann Pathol Lab Med. 2017; 4:A243–8.

58. Sunder KS, Khan MI. Role of fine needle aspiration cytology (FNAC) in diagnosis of thyroid lesions. J Contemp Med Dent. 2017; 5:30–4.
59. Garg S, Naik LP, Kothari KS, Fernandes GC, Agnihotri MA, Gokhale JC. Evaluation of thyroid nodules classified as Bethesda category III on FNAC. J Cytol. 2017; 34:5–9.

60. Kannan S, Raju N, Kekatpure V, et al. Improving Bethesda reporting in thyroid cytology: a team effort goes a long way and still miles to go. Indian J Endocrinol Metab. 2017; 21:277–81.

61. Mahajan S, Srinivasan R, Rajwanshi A, et al. Risk of malignancy and risk of neoplasia in the Bethesda indeterminate categories: study on 4,532 thyroid fine-needle aspirations from a single institution in India. Acta Cytol. 2017; 61:103–10.

62. Chandra S, Chandra H, Bisht SS. Malignancy rate in thyroid nodules categorized as atypia of undetermined significance or follicular lesion of undetermined significance: an institutional experience. J Cytol. 2017; 34:144–8.
63. Laishram RS, Zothanmawii T, Joute Z, Yasung P, Debnath K. The Bethesda system of reporting thyroid fine needle aspirates: a 2-year cytologic study in a tertiary care institute. J Med Soc. 2017; 31:3–7.

64. Witt BL, Schmidt RL. Rapid onsite evaluation improves the adequacy of fine-needle aspiration for thyroid lesions: a systematic review and meta-analysis. Thyroid. 2013; 23:428–35.

65. Shield PW, Cosier J, Ellerby G, Gartrell M, Papadimos D. Rapid on-site evaluation of fine needle aspiration specimens by cytology scientists: a review of 3032 specimens. Cytopathology. 2014; 25:322–9.

66. Pitman MB, Abele J, Ali SZ, et al. Techniques for thyroid FNA: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008; 36:407–24.

67. Keyhani E, Sharghi SA, Amini R, et al. Liquid base cytology in evaluation of thyroid nodules. J Diabetes Metab Disord. 2014; 13:82.

68. Prasad D, Bhaskar V, Kumar B. Study of utility of manual liquid-based cytology and conventional smears in the evaluation of various fine-needle aspiration samples. Paripex Indian J Res. 2017; 6:609–10.
69. Tripathy K, Misra A, Ghosh JK. Efficacy of liquid-based cytology versus conventional smears in FNA samples. J Cytol. 2015; 32:17–20.

70. Gupta V, Bhake A, Dayal S. Better thyroid cytopathology reporting and interpretation using different classification systems. Thyroid Res Pract. 2016; 13:110–4.

71. Bose S, Kapila K, Verma K. Medullary carcinoma of the thyroid: a cytological, immunocytochemical, and ultrastructural study. Diagn Cytopathol. 1992; 8:28–32.

72. Gupta A, Jain S, Khurana N, Kakar AK. Expression of p63 and Bcl-2 in malignant thyroid tumors and their correlation with other diagnostic immunocytochemical markers. J Clin Diagn Res. 2016; 10:EC04–8.

73. Tandon A, Paul TR, Singh R, Narendra AM. Synchronous thyroid involvement in plasma cell leukemia masquerading as Hashimoto’s thyroiditis: role of ancillary cytology techniques in diagnostic workup. Endocr Pathol. 2015; 26:324–7.

74. Goel MM, Budhwar P. Fine needle aspiration cytology and immunocytochemistry in tuberculous thyroiditis: a case report. Acta Cytol. 2008; 52:602–6.
75. Aron M, Kapila K, Verma K. Utility of galectin 3 expression in thyroid aspirates as a diagnostic marker in differentiating benign from malignant thyroid neoplasms. Indian J Pathol Microbiol. 2006; 49:376–80.
76. Jain D, Mathur SR, Guleria R, Iyer VK. Utility and pattern of positivity of p40 in the diagnosis of squamous cell carcinoma of the lung by cytology: the first study on fine needle aspiration smears. Cytopathology. 2014; 25:330–5.

77. Jain D, Mathur SR, Sharma MC, Iyer VK. Cytomorphology of sebaceous carcinoma with analysis of p40 antibody expression. Diagn Cytopathol. 2015; 43:456–61.

78. Vallonthaiel AG, Jain D, Singh V, et al. c-Myb overexpression in cytology smears of tracheobronchial and pulmonary adenoid cystic carcinomas. Acta Cytol. 2017; 61:77–83.

79. Roy M, Jain D, Bakhshi S, Mathur S, Iyer VK. Primary Langerhans cell histiocytosis of the thyroid gland: role of langerin in FNA cytological diagnosis. Cytopathology. 2015; 26:128–30.

80. Jain D, Mathur SR, Iyer VK. Cell blocks in cytopathology: a review of preparative methods, utility in diagnosis and role in ancillary studies. Cytopathology. 2014; 25:356–71.

81. Bhandari V, Yadav YK, Khanna G, Sharma M, Singh M. Efficacy of cytokeratin 19 expression on fine needle aspiration cell blocks in pre-operative diagnosis of malignant thyroid neoplasms. Clin Cancer Invest J. 2012; 1:212–6.
82. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; 26:1–133.
83. Reddy KH, Kumar DR, Punnoose AM, et al. Detection of BRAF mutations in different thyroid cancers of archival FNAC samples. Indian J Endocrinol Metab. 2013; 17(Suppl 1):S373–394.
84. Bychkov A. Prevalence of BRAFV600E mutation in Asian patients with thyroid cancer. Malays J Pathol. 2017; 39:95–6.
85. Katna R, Shet T, Sengar M, et al. Clinicopathologic study and outcome analysis of thyroid lymphomas: experience from a tertiary cancer center. Head Neck. 2013; 35:165–71.

86. Usha M, Kamath S, Sridhar M, Soman S. Primary thyroid lymphoma in the background of Hashimoto thyroiditis. Clin Cancer Invest J. 2015; 4:362–4.
87. Yeshvanth SK, Lakshminarayana KP, Upadhyaya VS, Shetty JK. Primary thyroid lymphoma arising from Hashimotos thyroiditis diagnosed by fine needle aspiration cytology. J Cancer Res Ther. 2012; 8:159–61.

88. Somani S, Kotwal N. Core needle biopsy: an additional diagnostic armamentarium. Thyroid Res Pract. 2016; 13:52–6.

89. Straccia P, Rossi ED, Bizzarro T, et al. A meta-analytic review of the Bethesda System for Reporting Thyroid Cytopathology: has the rate of malignancy in indeterminate lesions been underestimated? Cancer Cytopathol. 2015; 123:713–22.

90. Olson MT, Clark DP, Erozan YS, Ali SZ. Spectrum of risk of malignancy in subcategories of ‘atypia of undetermined significance’. Acta Cytol. 2011; 55:518–25.

91. Horne MJ, Chhieng DC, Theoharis C, et al. Thyroid follicular lesion of undetermined significance: evaluation of the risk of malignancy using the two-tier subclassification. Diagn Cytopathol. 2012; 40:410–5.

92. Kim SD, Han SH, Jeong WJ, Kim H, Ahn SH. Differences in clinical features between subcategories of “atypia/follicular lesion of undetermined significance”. Endocr Pathol. 2017; May. 9. [Epub]. https://doi.org/10.1007/s12022-017-9486-3.

93. Rosario PW, Calsolari MR. Importance of cytological subclassification of thyroid nodules with Bethesda category III cytology (AUS/FLUS) into architectural atypia only and nuclear atypia: a prospective study. Diagn Cytopathol. 2017; 45:604–7.

94. Layfield LJ, Baloch ZW, Esebua M, Kannuswamy R, Schmidt RL. Impact of the reclassification of the non-invasive follicular variant of papillary carcinoma as benign on the malignancy risk of the Bethesda System for Reporting Thyroid Cytopathology: a meta-analysis study. Acta Cytol. 2017; 61:187–93.

Table 1.
Review of the published studies on thyroid FNA in India
| No. | Study | Place | Needle size and technique | Wet fixation |
Staining technique used |
Reporting system followed | |
|---|---|---|---|---|---|---|---|
| Wet fixation | Air-dried smears | ||||||
| 1 | Mandreker et al. (1995) [13] | Goa | n/s | n/s | n/s | Unsatisfactory, benign, SFM, malignant | |
| 2 | Sirpal (1996) [14] | Delhi | 21G | n/s | Pap, H&E | Leishman-Giemsa | Malignant, non-neoplastic, FN, Hurthle cell tumors, thyroglossal cyst, extrathyroidal, inconclusive when unsatisfactory |
| 3 | Handa et al. (2008) [15] | Chandigarh | 23G ± aspiration, manuala | n/s | Pap, H&E | MGG | n/s |
| 4 | Guhamallick et al. (2008) [16] | Kolkata | 23–24G + aspiration | n/s | Pap, H&E | Leishman-Giemsa | Unsatisfactory, non-neoplastic, indeterminate, malignant |
| 5 | Gupta et al. (2010) [17] | Jammu and Kashmir | n/s | Ether-95% alcohol solution | Pap | n/s | Benign, FN, SFM, malignant |
| 6 | Bagga and Mahajan (2010) [18] | Haryana | 23–25G, non-aspirationa | 95% Ethanol | H&E | MGG | Unsatisfactory, benign, SFM, malignant |
| 7 | Sengupta et al. (2011) [19] | Bihar | 22–23G + aspirationb | n/s | n/s | MGG | Colloid goiter, granulomatous thyroiditis, FA, FC, anaplastic carcinoma |
| 8 | Renuka et al. (2012) [20] | Andhra Pradesh | 22G ± aspiration, USG in some | 95% Methanol | Pap, H&E | MGG | TBSRTC |
| 9 | Sharma and Mathur (2012) [21] | Rajasthan | 23G + aspiration | Ether-95% alcohol solution | Pap, H&E | Giemsa | Unsatisfactory, non-neoplastic, FN, SFM, malignant (RCP) |
| 10 | Patel et al. (2013) [22] | Gujarat | 23–24G ± aspirationa | 95% Ethanol | Pap, H&E | MGG | Non-neoplastic, neoplastic, others |
| 11 | Mondal et al. (2013) [23] | West Bengal | n/s, USG in somea | n/s | Pap | Leishman-Giemsa | TBSTRC |
| 12 | Kukar et al. (2013) [24] | Punjab | n/sa | 95% isopropanol | Pap, H&E | MGG | Non-neoplastic, neoplastic |
| 13 | Bhasin et al. (2013) [25] | Punjab | n/s | n/s | n/s; MGG as per figures | TBSRTC | |
| 14 | Borgohain et al. (2014) [26] | Assam | n/s | n/s | n/s | MGG | Non-neoplastic, neoplastic |
| 15 | Mangshetty et al. (2014) [27] | Karnataka | 22–24G + aspiration | Alcohol | Pap | MGG | Unsatisfactory, benign, malignant |
| 16 | Panchal et al. (2014) [28] | Maharashtra | 22/23G + aspiration | 95% Ethanol | Pap | n/s | Unsatisfactory, benign, SFM, malignant |
| 17 | Pathak et al. (2014) [29] | Delhi | n/s | n/s | Pap | MGG | TBSRTC |
| 18 | Sukumaran et al. (2014) [30] | Kerala | n/s | 95% alcohol | Pap | n/s | TBSRTC |
| 19 | Arul and Masilamani (2015) [31] | Tamil Nadu | n/s | n/s | n/s, H&E as per figures | TBSRTC | |
| 20 | Arul et al. (2015) [32] | Tamil Nadu | n/s, USG if small lesiona | n/s | H&E | MGG | TBSRTC |
| 21 | Sekhar et al. (2015) [33] | Karnataka | 23–24G + aspiration | 95% Ethanol | Pap, H&E | MGG | TBSRTC |
| 22 | Mehra and Verma (2015) [34] | Delhi | n/s, USG in all | n/s | Pap | MGG | TBSRTC |
| 23 | Agrawal et al. (2015) [35] | Uttar Pradesh | 23G + aspiration | 95% Ethanol | Pap, H&E | MGG | TBSRTC |
| 24 | Sharma (2015) [36] | Tamil Nadu | n/s, manual | n/s | n/s | Unsatisfactory, benign, follicular pattern lesions, suspicious (includes atypical), malignant | |
| 25 | Thakkar et al. (2015) [37] | Gujarat | 22/24G + aspirationa | n/s | H&E | MGG | TBSRTC |
| 26 | Garg et al. (2015) [38] | Gujarat | 23–24G + aspirationa | n/s | n/s | TBSRTC | |
| 27 | Kathirvel (2015) [39] | Tamil Nadu | 25–27G | 100% Isopropanol | H&E | TBSRTC | |
| 28 | Alagarsamy et al. (2015) [40] | Tamil Nadu | 23G ± aspiration | 100% Isopropanol | H&E | n/s | Colloid goiter, adenoma, carcinoma, others |
| 29 | Mamatha et al. (2015) [42] | Telangana | n/s | n/s | n/s | n/s | Unsatisfactory, colloid cyst/goiter, follicular lesions/ neoplasm, indeterminate, SFM, malignant as well as TBSRTC |
| 30 | Gupta et al. (2015) [43] | Uttar Pradesh | n/s | n/s | Pap | Diff-Quick | Histopathological equivalents as well as TBSRTC |
| 31 | Hathila et al. (2016) [41] | Gujarat | 23G, non-aspiration | 95% Ethanol | Pap, H&E | MGG | Benign, malignant |
| 32 | Shankar et al. (2016) [44] | Tamil Nadu | n/s + aspiration | n/s | Pap | n/s | TBSRTC |
| 33 | Prathima et al. (2016) [45] | Karnataka | n/s, USG in some | Alcohol | Pap, H&E | Giemsa | TBSRTC |
| 34 | Mehrotra et al. (2016) [46] | Karnataka | n/s ± aspiration, USG in some | 95% Ethanol | H&E | MGG | TBSRTC |
| 35 | Tagore et al. (2016) [47] | Karnataka | 22G + aspiration | Alcohol | Pap | MGG | TBSRTC |
| 36 | Kalita and Das (2016) [48] | Assam | 23G ± aspiration | n/s | n/s | MGG | TBSRTC |
| 37 | Bhartiya et al. (2016) [49] | Bihar | 23–24G + aspiration, USG in somea | Wet fixed | Pap | Leishman-Giemsa | TBSRTC |
| 38 | Kulkarni et al. (2016) [50] | Madhya Pradesh | n/s | n/s | Pap | n/s | TBSRTC |
| 39 | Lohiya et al. (2016) [51] | Rajasthan | 23/24G | n/s | n/s | MGG | TBSRTC |
| 40 | Kasliwal et al. (2016) [52] | 24–26G + aspiration | 95% Ethanol | H&E | MGG | TBSRTC | |
| 41 | Khatib et al. (2016) [53] | Maharashtra | n/s, USG if unsatisfactory | n/s | Pap | Giemsa | TBSRTC |
| 42 | Pantola et al. (2016) [54] | Tamil Nadu | 23G | 95% Alcohol | Pap, H&E | MGG | TBSRTC |
| 43 | Babu et al. (2016) [55] | Tamil Nadu | 23G | Ether-95% alcohol solution | Pap | n/s | Unsatisfactory, benign, malignant |
| 44 | Solanki et al. (2016) [56] | Rajasthan | n/s | n/s | H&E | MGG | TBSRTC |
| 45 | Aramani and Gururajaprasad (2017) [57] | Karnataka | 24–25G + aspiration | 95% Ethanol | Pap, H&E | MGG | Benign, malignant |
| 46 | Sunder and Khan (2017) [58],b | Telangana | 23/25/26G + aspiration | 95% Ethyl alcohol or isopropanol | Pap, H&E | MGG | Benign, malignant |
| 47 | Garg et al. (2017) [59] | Maharashtra | n/s ± aspiration, USG in all | n/s | Pap | MGG | TBSRTC |
| 48 | Kannan et al. (2017) [60] | Karnataka | n/sa,b | n/s | n/s | n/s | TBSRTC |
| 49 | Mahajan et al. (2017) [61] | Chandigarh | n/s | n/s | n/s | n/s | TBSRTC |
| 50 | Chandra et al. (2017) [62] | Uttarakhand | 26–28G | Alcohol | Pap, H&E | MGG | TBSRTC |
| 51 | Laishram et al. (2017) [63] | Manipur | n/s | n/s | n/s | MGG | TBSRTC |
FNA, fine needle aspiration; n/s, not specified; SFM, suspicious for malignancy; Pap, Papanicolaou stain; H&E, hematoxylin and eosin; FN, follicular neoplasm; MGG, May-Grünwald-Giemsa; FA, follicular adenoma; FC, follicular carcinoma; USG, ultrasound-guided aspirate; TBSRTC, The Bethesda System for Reporting Thyroid Cytopathology; RCP, Royal College of Pathologists guidelines.
Table 2.
Descriptive data with the risk of malignancy
| No. | Study | Thyroid FNA (operated nodules) |
Distribution of the Bethesda categories and corresponding risk of malignancy (%) |
|||||
|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | |||
| 1 | Mandreker et al. (1995) [13] | 1,992 (238) | 12.7 | 78.2 (5.5) | - | - | 7.6 (26.3) | 1.5 (91.7) |
| 2 | Sirpal (1996) [14] | 1,114a (128) | 0.6b (0) | 97.1 (0) | - | 1 (11.1) | - | 1.3 (100) |
| 3 | Handa et al. (2008) [15] | 434 (66) | 5.1 | 87.8 (1.9) | - | 3.2 (0) | - | 3.9 (100) |
| 4 | Guhamallick et al. (2008) [16] | 288 (75) | 13.5 | 68.4 (3.1) | - | 9.4 (30) | - | 8.7 (95.6) |
| 5 | Gupta et al. (2010) [17] | 75 (75) | - | 60 (6.7) | - | 24 (16.7) | 4 (0) | 12 (100) |
| 6 | Bagga and Mahajan (2010) [18] | 252 (32) | 1.6 | 90.5 | - | - | 6.7 | 1.2 |
| 7 | Renuka et al. (2012) [20] | 564 | 17 | 70.5 | 1.9 | 4.2 | 2.6 | 3.5 |
| 8 | Sharma and Mathur (2012) [21] | 94 (76) | 2.1 | 53.2 (0) | - | 35.1 (10) | 2.1 (100) | 7.4 (100) |
| 9 | Mondal et al. (2013) [23] | 1,020 (323) | 1.2 (0) | 87.5 (4.5) | 1 (20) | 4.2 (30.6) | 1.4 (75) | 4.7 (97.8) |
| 10 | Bhasin et al. (2013) [25] | 80 | 1.2 | 61.2 | 10 | 20 | 3.8 | 3.8 |
| 11 | Panchal et al. (2014) [28] | 300 (36) | - | 98.7 | - | - | 0.3 | 1 |
| 12 | Pathak et al. (2014) [29] | 454 | 25.7 | 59 | 6 | 4 | 1.8 | 3.5 |
| 13 | Sukumaran et al. (2014) [30] | 248 (248) | 6 (6.7) | 12.5 (12.9) | 4.4 (54.6) | 13.3 (87.9) | 4 (100) | 59.7 (100) |
| 14 | Arul and Masilamani (2015) [31] | 483 (209) | 5 (8.3) | 44.5 (1.1) | 2.9 (0) | 21.5 (11.5) | 15.3 (96.9) | 10.8 (100) |
| 15 | Arul et al. (2015) [32] | 603 (392) | 2.7 (0) | 65.2 (1.2) | 10 (24.4) | 10.6 (28.9) | 5.3 (70.8) | 6.3 (100) |
| 16 | Sekhar et al. (2015) [33] | 150 (64) | 2.6 (0) | 76.6 (0) | 0.7 | 12.7 (5.9) | 2.7 (100) | 4.7 (66.7) |
| 17 | Mehra and Verma (2015) [34] | 225 (40) | 7.2 (0) | 80 (13) | 4.9 (100)c | 2.2 (25) | 3.5 (50) | 2.2 (100) |
| 18 | Agrawal et al. (2015) [35] | 281 (134) | 2.5 (0) | 87.9 (1.8) | 3.9 | 2.5 (18.2) | 1.8 (80) | 1.4 (100) |
| 19 | Sharma (2015) [36] | 724 (724) | - | 87.7 (1.3) | - | - | 2.9 (52.4) | 9.4 (97.1) |
| 20 | Thakkar et al. (2015) [37] | 134 (24) | 4.5 (0) | 85.8 (0) | 0.7 | 7.5 (33.3) | 0.7 | 0.7 |
| 21 | Garg et al. (2015) [38] | 100 (60) | 6 (20) | 78 (0) | 4 (25) | 5 (20) | 3 (66.7) | 4 (100) |
| 22 | Kathirvel (2015) [39] | 59 | 15.3 | 16.9 | 15.3 | 16.9 | 15.3 | 20.3 |
| 23 | Mamatha et al. (2015) [42] | 240 (214) | 10.8 | 59.2 (0) | 4.2 (50) | 15 (6.7) | 4.2 (60) | 6.6 (100) |
| 24 | Gupta et al. (2015) [43] | 300 | 11 | 78 | 2 | 3 | 1 | 5 |
| 25 | Shankar et al. (2016) [44] | 402 (92) | 10.7 (0) | 81.6 (1.6) | 1.2 (0) | 1.7 (28.6) | 2 (71.4) | 2.7 (80) |
| 26 | Prathima et al. (2016) [45] | 178 (60) | 11.7 (33.3) | 77.5 (7.1) | 1.1 (50) | 3.9 (25) | 2.2 (66.7) | 3.3 (100) |
| 27 | Mehrotra et al. (2016) [46] | 175 (34) | 4.6 | 68.6 (0) | 5.7 | 17.1 (0) | 1.1c (0) | 2.9c (100) |
| 28 | Tagore et al. (2016) [47] | 100 | 3 | 81 | 0 | 9 | 3 | 4 |
| 29 | Kalita and Das (2016) [48] | 664 | 12 | 72.3 | 1.8 | 4.8 | 1.5 | 7.5 |
| 30 | Bhartiya et al. (2016) [49] | 238 (105) | 5.9 | 84 (2) | 1.3 | 2.9 (0) | 2.5 | 3.4 (100) |
| 31 | Kulkarni et al. (2016) [50] | 151 (16) | 11.2 (0) | 76.8 (0) | 0 | 9.3 (25) | 0.7 | 2 (100) |
| 32 | Lohiya et al. (2016) [51] | 250 | 4 | 88 | 2 | 1.6 | 0.8 | 3.6 |
| 33 | Kasliwal et al. (2016) [52] | 411 (97) | 0.5 | 94.2 (2.6) | 0 | 3.5 (22.2) | 0 | 1.7 (100) |
| 34 | Khatib et al. (2016) [53] | 287 (287) | 0.7 (0) | 87.8 (3.3) | 3.5 (20) | 4.2 (25) | 1.7 (80) | 2.1 (100) |
| 35 | Pantola et al. (2016) [54] | 218 (44) | 5.5 (0) | 69.3 (0) | 10.5 (8.3) | 8.2 (10) | 2.3 (100) | 4.1 (100) |
| 36 | Solanki et al. (2016) [56] | 1,287 (62) | 22 (18.2) | 73.9 (2.6) | 0.7 (0) | 1.5 (50) | 0.4 (50) | 1.3 (100) |
| 37 | Kannan et al. (2017) [60] | 404 (243) | 7.7 (28.6) | 40.8 (13) | 24.3 (41.7) | 10.6 (46.9) | 6.9 (96.3) | 9.7 (100) |
| 38 | Mahajan et al. (2017) [61] | 4,532 (335) | 3.5 (50) | 79.6 (7.8) | 2.5 (50) | 3.9 (23.6) | 0.5 (75) | 9.8 (85.4) |
| 39 | Chandra et al. (2017) [62] | 971d | 5.5 | 74.9 | 6.4 (51.4) | 2.6 | 3.2 | 7 |
| 40 | Laishram et al. (2017) [63] | 576 (11) | 5.2 | 89.9 | 0 | 2.2 (40) | 0.3 | 2.2 (100) |
Table 3.
Summary of meta-analysis
| Category |
ROM |
RON |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Studies included for ROM | Pooled ROM (95% CI, %) | I2 (%) | p-value | ROM as per TBSRTC (%) [5] | Studies included for RON | Pooled RON (95% CI, %) | I2 (%) | p-value | |
| I | 18 | 15 (6–24) | 11.8 | .34 | 1–4 | 15 | 34 (17–52) | 65.5 | .01 |
| II | 28 | 3 (2–4) | 54 | .00 | 0–3 | 23 | 8 (6–10) | 80.3 | .00 |
| III | 15 | 34 (23–45) | 57.9 | .01 | 5–15 | 11 | 62 (44–81) | 77.4 | .00 |
| IV | 27 | 26 (15–36) | 87.2 | .00 | 15–30 | 23 | 81 (73–89) | 77.2 | .00 |
| V | 21 | 69 (55–84) | 87.7 | .00 | 60–75 | 17 | 76 (62–90) | 64.1 | .00 |
| VI | 28 | 94 (89–98) | 56.3 | .03 | 97–99 | 24 | 95 (90–99) | 58.1 | .05 |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download