Abstract
Carcinosarcoma of the salivary gland is an extremely rare tumor that is composed of both malignant epithelial and mesenchymal components. Diagnosing carcinosarcoma with fine-needle aspiration cytology is challenging because of its overlapping cytomorphologic characteristics with other high-grade malignant salivary gland tumors. Among the many features, including pleomorphic oncocytoid epithelial components, necrotic background, and mitoses, recognizing the singly scattered atypical spindle cells is most essential in carcinosarcoma. We present a case of a 66-year-old male patient with characteristic features of carcinosarcoma, who was successfully treated by wide local excision and subsequent radiation therapy.
The term carcinosarcoma of the salivary gland, which means a tumor consisting of both carcinomatous and sarcomatous elements, was first used by King [1] in 1967. The three distinct histologic types of malignant mixed tumor are carcinoma ex pleomorphic adenoma, which accounts for most malignant mixed tumors, metastasizing mixed tumor, and carcinosarcoma, also known as true malignant tumor. To the best of our knowledge, approximately 60 cases of carcinosarcomas of the salivary glands have been reported in the English literature. Patients with carcinosarcoma of the salivary gland should be considered for additional chemotherapy or radiotherapy [2]. Its differential diagnosis is extremely important. However, making a correct diagnosis on an aspirated cytologic specimen is a challenge for pathologists because of various histologic features. Herein, we present a case of carcinosarcoma of the salivary gland diagnosed by preoperative fine-needle aspiration cytology (FNAC).
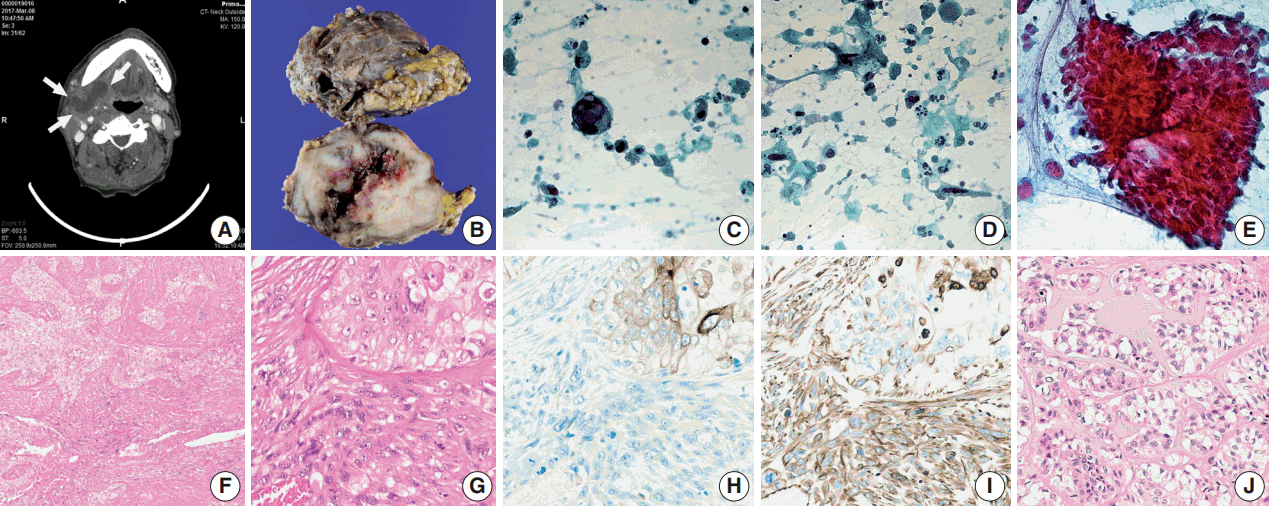
A 66-year-old man presented with a mass in the right submandibular gland that had been rapidly enlarging for several months. His medical history included hypertension, cardiovascular attack, and unstable angina. The computed tomography scan showed a 5-cm-sized movable mass with sialolithiasis in the right submandibular area (Fig. 1A). The FNAC specimen contained numerous single malignant epithelial cells that had marked nuclear pleomorphism, increased nuclear-cytoplasmic ratio, and coarse chromatin pattern with prominent nucleoli (Fig. 1C). Additionally, a few atypical mucin-containing cells, reminiscent of mucoepidermoid carcinoma, were found. Abundant necrotic debris and a mixture of inflammatory cells were scattered in the background of dispersed atypical spindle cells (Fig. 1D). There were sheet-like fragments, which showed squamous differentiation with a few mitosis in only one out of ten FNAC slides (Fig. 1E). The patient underwent surgery to remove the mass and stones at Gyeongsang National University Changwon Hospital.
Overall, the cut surface showed a relatively well-circumscribed, ivory, heterogeneous mass that measured 5.5 × 4 cm and extended to the extra-parenchymal area (Fig. 1B). Microscopically, the tumor was mainly composed of two components—undifferentiated carcinoma (UC) and undifferentiated pleomorphic sarcoma (UPS) with a central necrosis (Fig. 1F). Under higher magnification, the carcinomatous component was haphazardly arranged with numerous mitoses. The sarcomatous components were permeating to the UC (Fig. 1G). The immunohistochemical findings were in a sharp contrast in these two components. Carcinoma cells were positive for cytokeratin (Fig. 1H), whereas sarcoma cells were negative for cytokeratin and positive for vimentin (Fig. 1I). Focal areas mimicking epithelial-myoepithelial carcinoma were observed (Fig. 1J).
All dissected 11 lymph nodes had no metastatic focus. The patient was successfully managed by wide local excision and subsequent radiation therapy. This study was approved by the Institutional Review Board of Gyeongsang National University Changwon Hospital with a waiver of informed consent (GNUCH 2017-09-009).
FNAC is a simple, safe, cost-effective, well-tolerated, and in particular, minimally invasive method [3]. On average, the salivary gland FNAC has high specificity (97%), but the sensitivity is relatively low (80%) [4]. This means that the diagnosis on FNAC is very reliable, but the false-negative rate associated with FNAC (20%) may not be acceptable [4]. FNAC determines the extent of surgery needed after malignant tumor is diagnosed. It helps in deciding whether the facial nerve can be spared during the surgery and therefore, it is still important [3]. Diagnosing a high-grade salivary gland tumor, especially carcinosarcoma, on FNAC is challenging; thus, we should approach more systematically. Griffith et al. [5] proposed a risk stratification of FNAC in salivary gland tumors, which are classified as non-neoplastic and neoplastic. Among the neoplastic lesions, they proposed using the term oncocytoid and basaloid neoplasm rather than pleomorphic adenoma and Warthin tumor, the two most common tumors of the salivary gland. They also subdivided the oncocytoid and basaloid groups based on their nuclear grade (as monomorphic and pleomorphic groups), background characteristics, and stromal features. The pleomorphic oncocytoid neoplasm group was universally high-grade malignancies (21/21) and most of these 21 cases were of salivary duct carcinomas and several other high-grade carcinoma types including three high-grade mucoepidermoid carcinoma, one poorly differentiated carcinoma, and one UC [5].
Based on the criteria of abundant cytoplasm, high nuclear grade with pleomorphism and hyperchromasia, and increased mitotic activity, the “pleomorphic oncocytoid neoplasm” was very identical to the carcinomatous components in our case. In addition, some other findings were observed, including isolated giant cells with vesicular nuclei and macronucleoli and isolated atypical spindle cells with hyperchromatic nuclei. These two different cells were classified as atypical because they had variations in size and shape more than three times the normal [6]. When FNAC findings were correlated with histological findings, the giant epithelial cells seemed to have come from the UC component, and the atypical spindle cells, either isolated or clustered with epithelial cells, seemed to have been exfoliated from the UPS component. In histologic section, UPS and UC were intermingled, showing different patterns of immunohistochemical staining for cytokeratin and vimentin, which is the key finding in confirming carcinosarcoma.
Because there were squamoid clusters without keratinization in the FNA specimen, mucoepidermoid carcinoma (MEC) with squamous elements and moderate to poorly differentiated squamous cell carcinoma (SCC) were included in differential diagnosis. If it were MEC, there must be numerous tumor cell clusters due to its hard and solid consistency in high-grade types [6]. Also, non-keratinizing, moderate to poorly differentiated SCCs usually exfoliate in clusters or sheets. Rapidly growing tumors like our case frequently produce central necrosis and often show cellular debris with necrotic background in their aspirates [6]. In our case, there were plenty of individual pleomorphic cells in abundant necrotic background. Only up to 23 cell clusters were contained in 10 aspirated slides. We suggest that more aggressive tumors including malignant mixed tumor and carcinosarcoma must be considered, if there are fewer carcinomatous clusters and abundant individual pleomorphic cells in necrotic background.
The sarcomatous components in our case occupied nearly 40% of the total tumor volume. However, these components were hardly seen in the FNAC. Both isolated spindle cells and sheet-like tissue fragments were found in one out of 10 FNAC slides. We hypothesized that matrix-forming characteristics of carcinosarcoma might have affected the hypocellularity of sarcomatous cells in the FNAC specimen. The malignant mesenchymal elements of carcinosarcomas are most commonly in the form of chondrosarcoma [2,7,8]. In the present case, however, features of chondrosarcoma were not observed in both cytologic and surgical specimens. In addition, tumor cells in sheets were not as pleomorphic as the isolated atypical spindle cells in the FNAC specimen. We should be concentrating on the scattered atypical cells or matrix-forming cells when we approach high-grade pleomorphic salivary gland tumors in FNAC. Frequently, isolated cells, which accurately reveal their characteristic morphologic features, are more important than three-dimensional clusters in cytologic specimens.
The histogenesis and pathogenesis of the carcinosarcoma are still under discussion. Some authors insist that pleomorphic adenoma and carcinosarcomas may share a common precursor cell in which the myoepithelial cell is a major component in their development [8,9]. In the present case, approximately 20% of the total volume of epithelial myoepithelial carcinoma-mimicking area was observed in the histologic specimen. However, these components did not show any immunoactivities for smooth muscle actin and S100, a marker of myoepithelial cells. Furthermore, histologic evidence of a preexisting or coexisting pleomorphic adenoma was not observed. These findings may indirectly prove that myoepithelial cells may not be the sole origin of carcinosarcoma. We agree with Kwon and Gu [10] that the primitive mesenchymal cells, which can be differentiated in diverse directions, may contribute to the development of different types of sarcomas in carcinosarcomas; thus, they have heterogeneous combinations of both epithelial and mesenchymal components [7].
Although carcinosarcoma is an extremely rare tumor, pathologists should be aware of this entity because the diagnosis of carcinosarcoma warrants concurrent radiation therapy extended to regional lymph nodes even if those lymph nodes are not metastatic.
We described the cytologic features of carcinosarcoma arising in the submandibular salivary gland. In FNAC of the salivary gland tumor, carcinosarcoma should be considered if atypical spindle cells and highly pleomorphic epithelial cells are identified in abundant necrotic background.
REFERENCES
1. King OH Jr. Carcinosarcoma of accessory salivary gland: first report of a case. Oral Surg Oral Med Oral Pathol. 1967; 23:651–9.
3. Schmidt RL, Hall BJ, Wilson AR, Layfield LJ. A systematic review and meta-analysis of the diagnostic accuracy of fine-needle aspiration cytology for parotid gland lesions. Am J Clin Pathol. 2011; 136:45–59.

4. Schmidt RL, Hunt JP, Hall BJ, Wilson AR, Layfield LJ. A systematic review and meta-analysis of the diagnostic accuracy of frozen section for parotid gland lesions. Am J Clin Pathol. 2011; 136:729–38.

5. Griffith CC, Pai RK, Schneider F, et al. Salivary gland tumor fine-needle aspiration cytology: a proposal for a risk stratification classification. Am J Clin Pathol. 2015; 143:839–53.
6. Naib ZM. Cytopathology. 4th ed. New York: Little Brown & Company;1996.
7. Marcotullio D, de Vincentiis M, Iannella G, Cerbelli B, Magliulo G. Mucoepidermoid carcinoma associated with osteosarcoma in a true malignant mixed tumor of the submandibular region. Case Rep Otolaryngol. 2015; 2015:694684.

8. Stephen J, Batsakis JG, Luna MA, von der Heyden U, Byers RM. True malignant mixed tumors (carcinosarcoma) of salivary glands. Oral Surg Oral Med Oral Pathol. 1986; 61:597–602.

9. Bleiweiss IJ, Huvos AG, Lara J, Strong EW. Carcinosarcoma of the submandibular salivary gland. Immunohistochemical findings. Cancer. 1992; 69:2031–5.

10. Kwon MY, Gu M. True malignant mixed tumor (carcinosarcoma) of parotid gland with unusual mesenchymal component: a case report and review of the literature. Arch Pathol Lab Med. 2001; 125:812–5.
Fig. 1.
Computed tomography image of the patient and gross examination, fine-needle aspiration specimen, and microscopic and immunohistochemical findings of carcinosarcoma. (A) Computed tomography scan shows a movable mass with sialolithiasis in the right submandibular area. (B) A well-circumscribed, ivory, heterogeneous mass is extended to the extra-parenchymal area. (C) A single malignant epithelial cell with marked nuclear pleomorphism, increased nuclear-cytoplasmic ratio, coarse chromatin pattern, and prominent nucleoli. (D) Abundant necrotic debris and a mixture of inflammatory cells are scattered in the background of dispersed atypical spindle cells. (E) Sheet-like fragments show squamous differentiation with a few mitosis. (F) Tumor is mainly composed of two components-undifferentiated carcinoma and undifferentiated pleomorphic sarcoma. (G) Under higher magnification, the carcinomatous component is haphazardly arranged with numerous mitoses and the sarcomatous components are permeating to the undifferentiated carcinoma. (H) Carcinoma cells are positive for cytokeratin. (I) Sarcoma cells are positive for vimentin. (J) Focal area mimicking epithelial-myoepithelial carcinoma.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download