This article has been
cited by other articles in ScienceCentral.
Abstract
Lymphomas arising in the central nervous system (CNS) of immunocompromised hosts are most commonly non-Hodgkin’s lymphomas and are highly associated with Epstein-Barr virus (EBV). Here we report an autopsy case of EBV-associated CNS diffuse large B-cell lymphoma (DLBCL) in a host suffering from systemic lupus erythematosus who underwent immunosuppressive therapy. After autopsy, EBV-associated CNS DLBCL as well as pulmonary mixed aspergillosis and Pneumocystis jirovecii pneumonia were added to the cause of clinical manifestations of complicated pneumonia and cerebral hemorrhage in this immunocompromised patient. In conclusion, complex disease processes were revealed by autopsy in this case, indicating that the clinicopathological correlations observed through autopsy can improve our understanding of disease progression and contribute to the management of similar patients in the future.
Go to :

Keywords: Autopsy, Central nervous system, Lymphoma, Epstein-Barr virus, Immunocompromised host
Lymphomas arising in an immunocompromised host are most commonly non-Hodgkin’s lymphoma, which is associated with Epstein-Barr virus (EBV) and can appear in the central nervous system (CNS) [
1,
2]. However, even when lymphoma is localized to the CNS, if the host is immunodeficient patients this does not represent primary CNS lymphoma (PCNSL), because the 2008 World Health Organization (WHO) classification of hematopoietic and lymphoid tissues limits PCNSL to diffuse large B-cell lymphoma (DLBCL) in immunocompetent patients [
3]. Therefore, PCNSL cannot be diagnosed in the patients with a history of underlying autoimmune diseases, organ transplantation, or immunosuppressive therapy. In addition, primary T-cell, NK-T cell or intravascular large B-cell lymphomas occurring in the CNS are not PCNSL, because they are not DLBCL. While PCNSL accounts for 2.4%–3.0% of all CNS tumors and comprises more than 95% of CNS lymphomas, EBV-associated CNS lymphoma (CNSL) is extremely rare [
4]. The majority of PCNSLs are sporadic and non-EBV–associated, and the incidence increases with age. Few CNSLs are related to immunocompromised states such as human immunodeficiency virus infection and iatrogenic immunosuppression following organ transplantation [
5]. In the latter cases, EBV infection is the most common cause of CNSL [
6].
We report a case of an immunocompromised patient diagnosed with EBV-associated CNSL after autopsy, focusing on the clinicopatholgical features of CNSL in immunocompromised patients. This case report followed the principles of the World Medical Association Declaration of Helsinki and it was approved by the Institutional Review Board of SNUH (H-1305-625-491 and IRB No. H-1506-080-681) with a waiver of informed consent.
CASE REPORT
The patient is a 60-year-old woman with a 2-year history of systemic lupus erythematosus (SLE) treated with prednisolone. Twelve years ago, she donated her left kidney to her daughter, who suffered from SLE for 12 years. She also had history of splenectomy due to idiopathic thrombocytopenic purpura (ITP) 8 years ago. One year ago, she underwent segmental resection of the small bowel due to cytomegalovirus (CMV) enteritis with perforation possibly related to steroid medication for SLE. Five months prior, she presented with left-side weakness and seizure. Magnetic resonance imaging (MRI) of the brain revealed multiple acute and subacute infarct-like lesions with hemorrhagic transformation of the left frontal and fronto-parietal lobe lesions (
Figs. 1,
2). Two months before death, she experienced fever relapses; at that time, chest imaging (chest posteroanterior) revealed patchy ground glass opacities of the bilateral upper lobes, indicating aggravated diffuse pneumonia (
Fig. 3). On last admission, liver function test revealed abnormalities: aspartate aminotransferase, 125 IU/L (normal, 0 to 40 IU/L); alanine aminotransferase, 57 IU/L (normal, 0 to 40 IU/L); and total bilirubin, 1.1 mg/dL (normal, 0.2 to 1.2 mg/dL). The patient’s general condition worsened and she died in December, 2012. A limited autopsy was performed confined to the brain, right lung and liver.
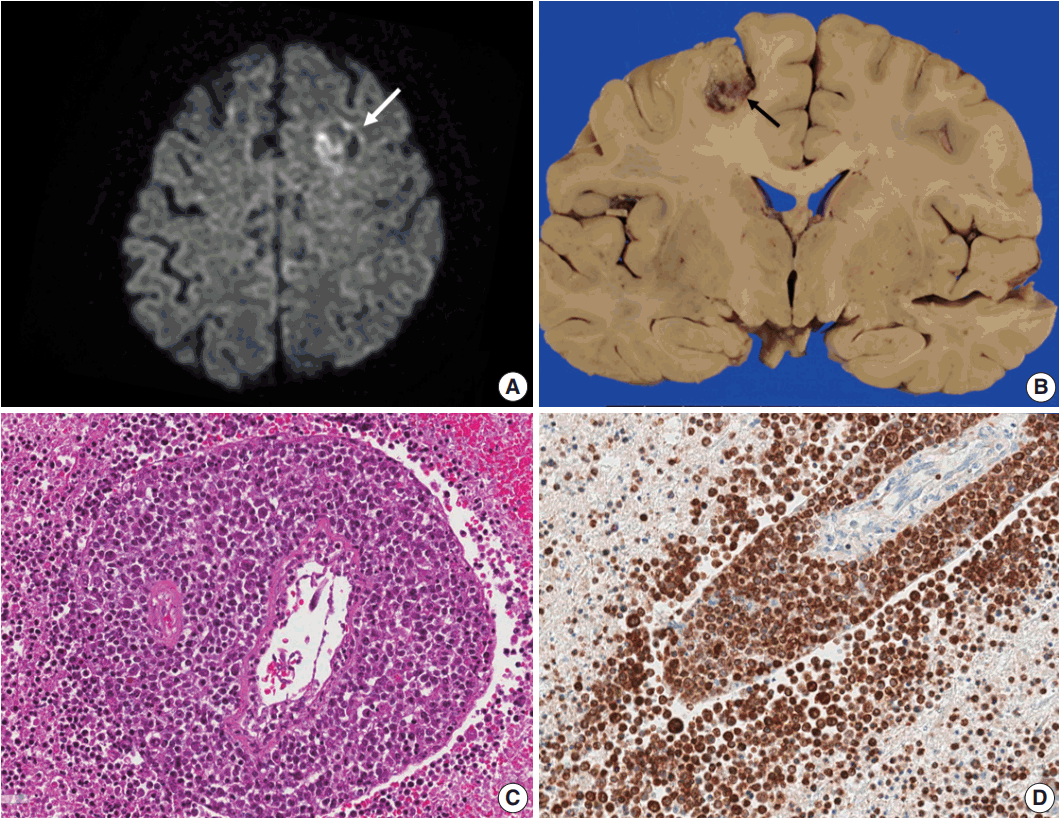 | Fig. 1.(A) Brain computed tomography shows a round enhanced lesion (arrow) in the left frontal area. (B) The cut surface of the brain shows a relatively well-demarcated friable lesion with hemorrhage and necrosis (arrow) in the left frontal cortex and white matter. (C) On light microscopy the tumor is composed of sheets of large atypical lymphocytes with angiocentricity. (D) CD20 immunostaining reveals that neoplastic lymphocytes are robustly positive. 
|
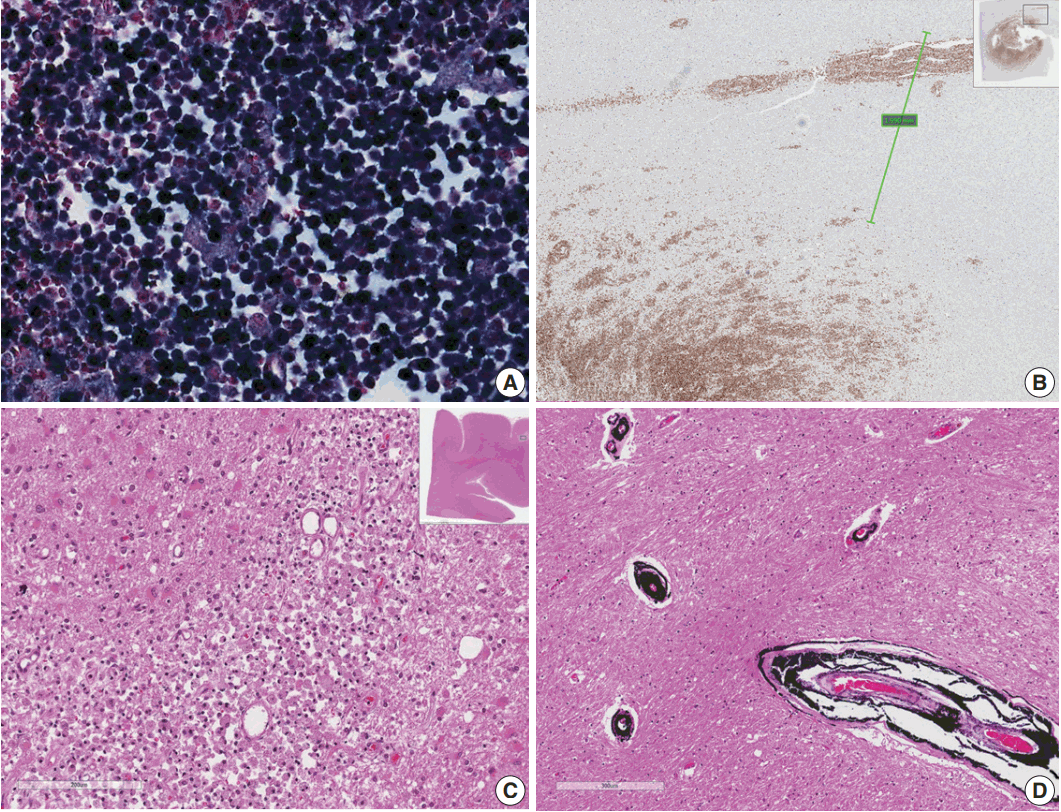 | Fig. 2.(A) Epstein-Barr virus (EBV)-encoded small RNA in situ hybridization shows that nearly 100% of tumor cells are positive for EBV. (B) CD20 immunostaining delineates individual infiltrating tumor cells and perivascular spread of tumor cells along blood vessels. The largest distance from the margin of the main mass is approximately 3 mm. (C) An infarct was found in the right frontal lobe, demonstrating rarefied brain with vascular proliferation, foamy macrophage infiltration and perilesional gemistocytic reactive gliosis. (D) The internal capsule exhibits thick perivascular calcifications. 
|
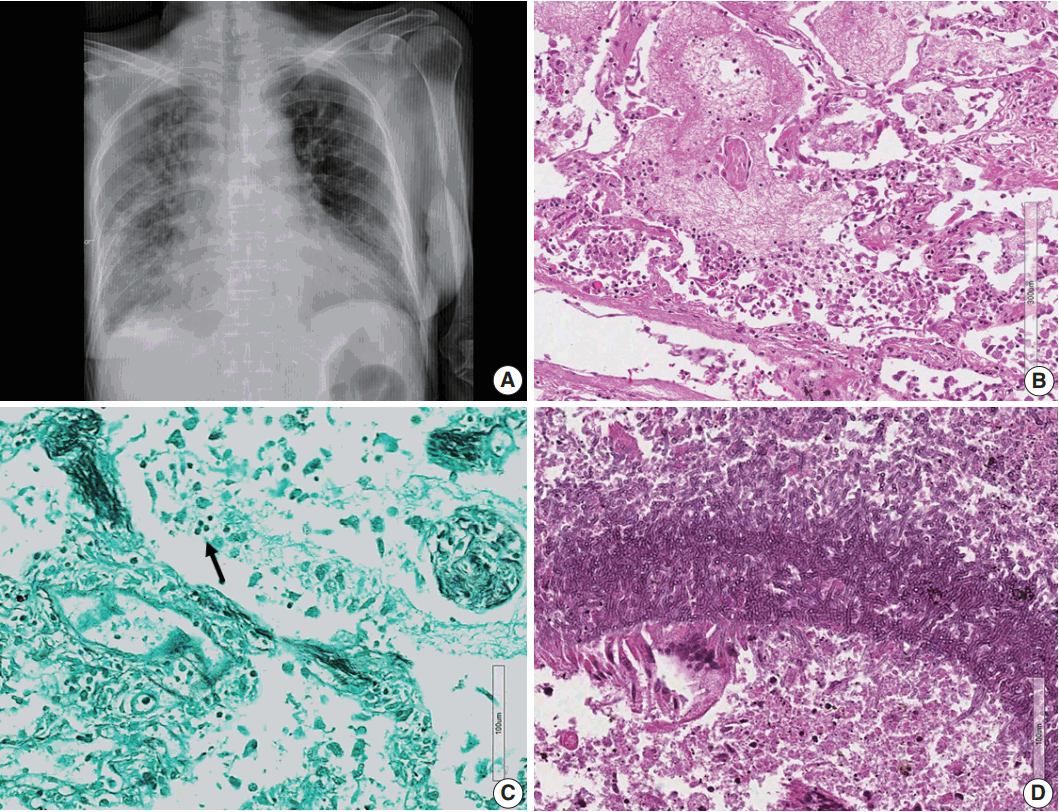 | Fig. 3.(A) Chest posteroanterior view shows diffuse haziness of both lungs with marked interstitial and right pleural effusion. (B) Microscopically, the lung shows diffuse alveolar damage, frothy intra-alveolar spaces, detached alveolar lining cells and many macrophages. (C) Gomori methenamine silver staining reveals tiny black oval-to-round Pneumocystis jirovecii organisms (arrow) in the intra-alveolar space. (D) Lung tissue shows a cavitary lesion with a fungus ball of aspergillosis (Periodic acid–Schiff staining). 
|
On autopsy, the whole brain was removed, which weighed 1,230 g in a fresh state. Both cerebral hemispheres were symmetric and dura and meninges were unremarkable. There was a 2.0 × 1.7 × 1.2-cm-sized hemorrhagic lesion on the left frontoparietal area of the coronal section (
Fig. 1). Right pneumonectomy was carried out (960 g in the fresh state). There were adhesions between the chest wall and parietal pleura. Patchy consolidations were found in the right upper lobe and a hemorrhagic lesion was found in the right lower lobe. Wedge resection of bilateral lobes of the liver revealed a clear external surface and a diffusely greenish bile-tinged cut surface. Cirrhosis was absent.
Microscopically, the frontal lobe had a small infarct, but the larger lesion on the left frontoparietal lobe of the brain was DLBCL, showing robust CD20 positivity and 100% EBV positivity of lymphoma cells on EBV-encoded small RNA
in situ hybridization (
Fig. 2). The margin of the lymphoma was relatively well demarcated, but the cells were also infiltrating blood vessels up to 2 mm from the main tumor margin (
Fig. 2). There were tiny clusters of lymphoma cells in the right frontal, upper midbrain and lower medulla oblongata. The right upper lobe of the lung showed invasive aspergillosis and
Pneumocystis jirovecii pneumonia (
Fig. 3). There was alveolar hemorrhage in the right lower lobe. The liver had bile plugs in the interlobular bile ducts with bile ductular proliferation consistent with intrahepatic cholestasis, but neither inflammatory cell infiltration nor any fungal or viral organism was present.
Go to :

DISCUSSION
There are two kinds of CNS DLBCL; one appears in immunocompetent patients and the other in immunodeficient patients. Primary CNS DLBCL in immunocompetent hosts are designated as PCNSLs by the WHO classification of tumors of hematopoietic and lymphoid tissue in 2008 [
3]. Primary CNS DLBCL exhibits specific gene expression and genomic profiles that differ from extra- CNS DLBCL, and the patients are managed with different protocols [
7]. The most significantly upregulated gene is extracellular matrix (ECM)-related osteopontin (secreted phosphoprotein 1,
SPP1). This ECM- and adhesion-related pathways may be related to the biologic characteristic of PCNSL, such as CNS tropism [
7].
Immunodeficiency-related lymphoma of the CNS differs from PCNSL of immunocompetent people based on its EBV positivity and tendency toward multifocality [
5,
8]. In general, CNS is one of the common site of EBV-associated DLBCLs in immunocompromised patients [
9]. There are two kinds of EBV-associated CNS DLBCL: age-related CNS DLBCL occurring in immunocompetent elderly patients (≥ 65 years old) and immunodeficiencyassociated CNS DLBCL [
1]. According to one study, the incidence of age-related EBV-associated CNSL without congenital or acquired immunodeficiency was reported to be about 4% (5/134). Primary CNS EBV-associated DLBCL in the elderly was higher in Asian countries than in Western countries [
2].
The prognosis of CNSL is very poor in both immunocompetent and immunocompromised hosts [
10]. Accumulated clinical experience over the past decade has proven that first-line treatment of PCNSL patients should avoid whole brain radiation. One approach includes combined induction immunotherapy with methotrexate, temozolomide, and rituximab followed by consolidative infusional etoposide plus high-dose cytarabine administered during the first complete remission [
11].
Here, we report a case of autopsy-proven EBV-associated CNSL arising in an immunocompromised host. The pre-autopsy clinical diagnosis was underlying SLE and immunosuppressive therapy-related intestinal CMV enteritis, and intrahepatic cholestasis as well as suspected brain infarcts in the right frontal and frontoparietal lobes. In addition to preautopsy clinical diagnosis, EBV-associated CNS DLBCL and pulmonary aspergillus and P. jirovecii pneumonia were found on autopsy.
The pathology of the liver suggests extrahepatic occlusion because bile plugs and bile duct proliferation were found. The patient had a CMV enteritis with perforation in the small bowel. CMV usually does not affect healthy people, but does symptomatically infect immunocompromised hosts. In this case, the patient had been taking prednisolone for a couple of years because of SLE and ITP; therefore, she is presumed to have had steroidinduced immunosuppression. Invasive aspergillosis and P. jirovecii pneumonia are also infectious diseases related to immunocompromised status.
Diagnosis of CNSL can be difficult because MRI features overlap with other pathologies [
12]. In our case, the radiologic report from brain MRI suggested embolic infarctions. Since the majority of CNS lymphomas are diagnosed via stereotactic biopsy or, less commonly, by flow cytometric analysis of cerebrospinal fluid lymphocytes, the detailed pathology and appearance of infiltrations remain unclear. Therefore, our case is important because we were able to examine the lesions in detail. In this autopsy case, the gross margins of the main CNSL mass were relatively well demarcated, but tumors had spread to other areas as tiny clusters of lymphoma cells.
The tumor cells were robustly positive for CD20 and Bcl-2; however, they were negative for CD3, Bcl-6, CD10, MUM-1, and CMV. Bcl-6 and CD10 are usually positive in germinal center type DLBCL and Bcl-6 can be considered as a good prognostic marker, but our case was negative for these two markers [
13]. MUM-1 is usually positive in activated B-cell type of DLBCL [
14], but it was also negative in our case.
Radiologically, differential diagnosis of primary CNS DLBCL may include CNS embryonal tumors (previously called primitive neuroectodermal tumors) and high-grade gliomas, because both show high cellularity and necrosis. Pathological diagnosis is not difficult with the help of immunohistochemistry [
1].
In conclusion, we report an autopsy case of EBV-associated CNS-DLBCL in an immunocompromised host. The patient suffered from SLE for 2 years and had a history of splenectomy due to ITP, small bowel resection due to CMV enteritis and complications of bowel perforation, and complex pneumonia, which was associated with long-term steroid therapy for SLE. Pre-mortem MRI findings of CNS lesions were suspicious of infarcts, but the lesions were found to be EBV-associated DLBCL on pathology. The patient’s pulmonary lesions represented aspergillosis and P. jirovecii pneumonia. Therefore, the major pathology of this complicated case involving an immunocompromised host was correctly diagnosed on autopsy. Despite advances in diagnostic options and technology, autopsy remains an essential tool for determining cause of death and obtaining important pathologic information.
Go to :








 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download