In the present study, we analyzed and evaluated the diagnostic utility of prostatic and urothelial immunohistochemical markers in PAC and UC with variable differentiation.
DISCUSSION
Although the pathologic identification of PAC and UC using hematoxylin and eosin staining is not difficult in most cases, some cases may present a challenging diagnosis because the histologic appearance of poorly differentiated PAC can be very similar to that of high-grade UC [
3].
High-grade PAC may have enlarged nuclei and prominent nucleoli similar to UC, but little variability in the nuclear size or shape is generally observed in PAC compared with UC [
2,
3]. Additionally, even in high-grade PAC, there are few mitosis and pleomorphism compared with high-grade UC [
3]. Although high-grade UC commonly exhibits more pronounced pleomorphism compared with PAC [
2,
3], there have been cases of high-grade UC that were indistinguishable from high-grade PAC in terms of pleomorphism and cytologic atypia [
3]. UC tends to grow in nests and often shows conspicuous squamous differentiation and glassy eosinophilic cytoplasm. In contrast, the cytoplasm of PAC is generally pale and foamy [
3]. Additionally, the findings of focal cribriform glandular differentiation or infiltrating cords of cells are more typical features of PAC than UC [
2,
3]. As the findings with routine hematoxylin and eosin staining may overlap, immunohistochemical staining may help solve the diagnostic dilemma [
3]. Particularly, in poorly differentiated carcinomas involving both the prostate and bladder without any glandular differentiation, the pathology of the case should be evaluated immunohistochemically (
Table 4).
Table 4.
Reported sensitivities of immunohistochemical markers for PAC and UC
|
Immunohistochemical marker |
Current study (2016)
|
Chuang et al. (2007) [3]
|
Kunju et al. (2006) [6]
|
Mhawech et al. (2002) [5]
|
Genega et al. (2000) [10]
|
|
PAC |
UC |
PAC |
UC |
PAC |
UC |
PAC |
UC |
PAC |
UC |
|
PSA |
111/111 (100) |
16/138 (11.6) |
37/38 (97.4) |
0/35 (0) |
40/42 (95.2) |
0/36 (0) |
34/40 (85.0) |
0/45 (0) |
32/34 (94.1) |
0/46 (0) |
|
PSMA |
93/111 (83.8) |
1/138 (0.7) |
35/38 (92.1) |
0/35 (0) |
- |
- |
- |
- |
- |
- |
|
PAP |
102/111 (91.9) |
26/138 (18.8) |
- |
- |
40/42 (95.2) |
4/36 (11.1) |
38/40 (95.0) |
0/45 (0) |
32/34 (94.1) |
0/46 (0) |
|
P501s |
104/111 (93.7) |
1/138 (0.7) |
38/38 (100) |
2/35 (5.7) |
- |
- |
- |
- |
- |
- |
|
NKX3.1 |
98/111 (88.3) |
2/138 (1.4) |
36/38 (94.7) |
0/35 (0) |
- |
- |
- |
|
- |
- |
|
AMACR |
74/111 (66.7) |
12/138 (8.7) |
- |
- |
37/42 (88.1) |
13/36 (36.1) |
- |
- |
- |
- |
|
CK34βE12 |
2/111 (1.8) |
104/138 (75.4) |
3/38 (7.9) |
32/35 (91.4) |
1/42 (2.4) |
35/36 (97.2) |
- |
- |
2/34 (5.9) |
30/46 (65.2) |
|
p63 |
0/111 (0) |
102/138 (73.9) |
0/38 (0) |
29/35 (82.9) |
0/42 (0) |
33/36 (91.7) |
- |
- |
- |
- |
|
TM |
0/111 (0) |
63/138 (45.7) |
2/38 (5.3) |
24/35 (68.6) |
- |
- |
0/40 (0) |
22/45 (48.8) |
- |
- |
|
S100P |
4/111 (3.6) |
31/138 (22.5) |
3/38 (7.9) |
25/35 (71.4) |
- |
- |
- |
- |
- |
- |
|
GATA3 |
0/111 (0) |
117/138 (95.9) |
- |
- |
- |
- |
- |
- |
- |
- |
|
CK7 |
- |
- |
- |
- |
4/42 (9.5) |
34/36 (94.4) |
11/40 (27.5) |
39/45 (86.6) |
4/34 (11.8) |
38/46 (82.6) |
|
CK20 |
- |
- |
- |
- |
2/42 (4.8) |
19/36 (52.8) |
4/40 (10.0) |
30/45 (66.6) |
8/34 (23.5) |
10/46 (21.7) |
|
Uroplakin III |
- |
- |
- |
- |
- |
- |
0/40 (0) |
27/45 (60) |
- |
- |

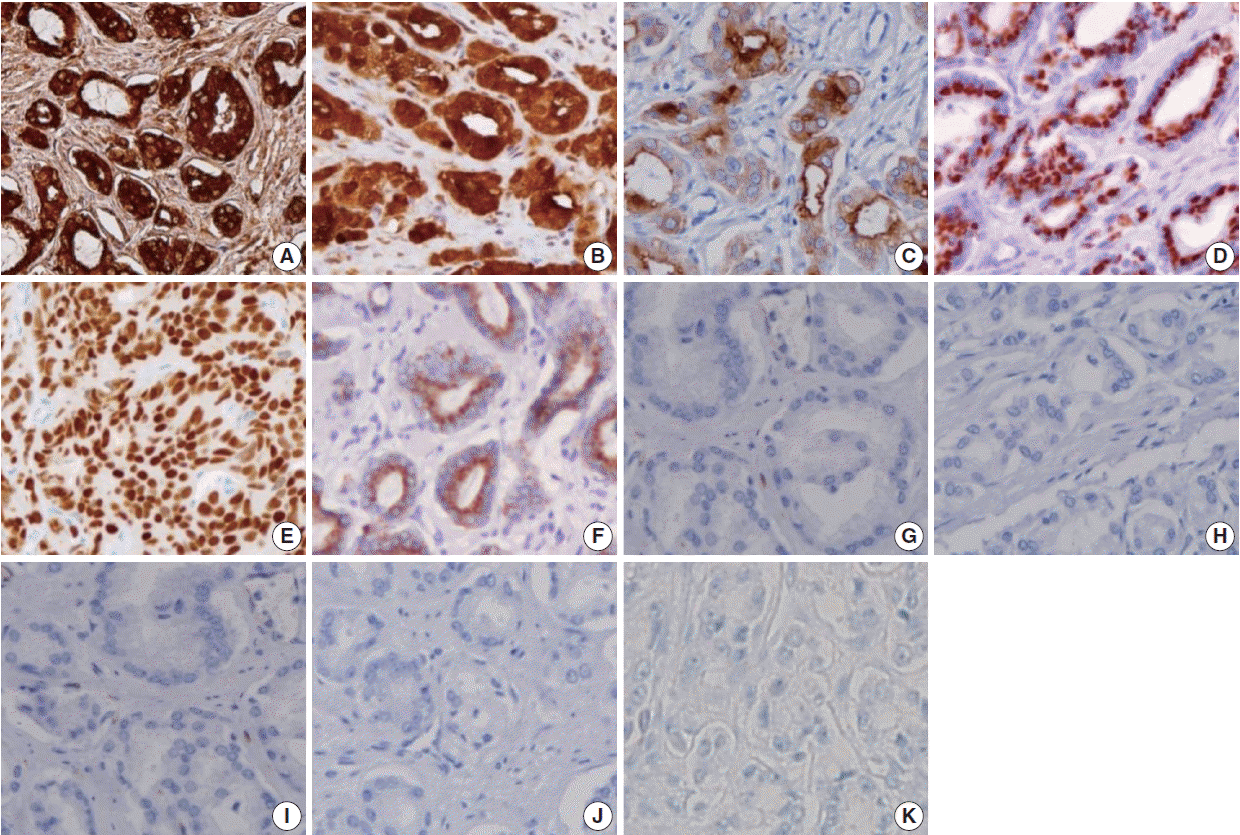
PSA, a serine protease member of the human glandular kallikrein family, is almost exclusively synthesized in the prostate ductal and acinar epithelium, making it a highly specific marker for the prostatic lineage [
2]. However, PSA has also been reported to be present in some non-prostatic tissue, such as the urethral, periurethral, and perianal glands [
4]. Extraprostatic neoplasms that frequently express PSA include urethral and periurethral adenocarcinoma, cloacogenic carcinoma, salivary gland pleomorphic adenoma, salivary duct carcinoma, and rare breast carcinomas [
8]. PSA has been shown to be a highly specific marker, but some authors suggest that there is an inverse correlation between the Gleason score and PSA staining intensity [
9]. Previous studies have reported that high-grade PAC that was completely negative for PSA stain ranged from 3% to 27% [
3,
5,
6,
9,
10]. However, in our study, no PAC specimens were devoid of PSA expression, including high-grade PAC, with 100% sensitivity. Therefore, PSA expression is very useful and valuable for clarifying the prostatic origin of tumors.
PSMA, a 750 amino acid type II membrane glycoprotein, is expressed by benign and malignant prostatic epithelial cells, with stronger staining observed in the latter [
11]. Although PSMA is a very specific marker of prostatic lineage, it is also expressed in non-prostatic tissues, such as the duodenal mucosa, neuroendocrine cells of colonic crypts, endothelial cells of some neoplasms, and proximal renal tubules [
12,
13]. Some studies have reported an inverse correlation between PSMA staining and the Gleason score [
11,
12]. The sensitivities of PSMA for PAC ranged from 86.8% to 100% in various studies [
3,
12-
15]. In our study, the sensitivity of PSMA in PAC (83.8%) was lower than PSA, but its specificity (99.3%) was higher than PSA. PSMA has been reported to stain 11% of urinary bladder adenocarcinomas, a fact worth noting [
16]. We detected scattered patterns of positive PSMA staining in only one from 138 cases (0.7%) of UC.
PAP is an early prostatic marker used to confirm the diagnosis of PAC5 [
15], and remains a specific marker for prostate tissue. Mhawech
et al. [
5] reported that 87% of high-grade PAC showed immunopositivity for PAP and observed an inverse correlation between the Gleason score and PAP staining. In this study, PAP was stained in 91.9% of PAC and showed a relative lack of specificity compared with PSMA (81.2% vs 99.3%), with a more variable staining pattern. Monoclonal antibodies to PAP have been reported to have lower sensitivities than their polyclonal counterparts but be more specific [
2]. PAP staining has been known to be consistently negative in UC [
5,
10,
17], but a recent study reported immunopositivity in 11.1% of UC [
6]. Unexpectedly, we also detected PAP staining with a scattered pattern in 26 of 138 cases of UC (18.8%).
P501s, a 553-amino acid protein located in the Golgi complex, is a newer prostate-specific protein identified by a combination of high-throughput microarray screening with cDNA subtraction [
18]. P501s is expressed by benign and malignant prostatic epithelium and has not been detected in the urothelium or non-prostatic tissue [
19]. P501s was reported to be expressed in 94% of a total of observed 113 PAC cases, independent of the metastatic status and Gleason score [
19]. Chuang
et al. [
3] reported that P501s was expressed in all 38 high-grade PAC cases. In the current study, P501s showed high sensitivity (93.7%) and specificity (99.3%) for PAC, and only one of 138 cases of UC (0.7%) was positive for that marker. To date, P501s expression has not been shown in tumors except PAC, making it of great utility in differentiating poorly differentiated PAC from high-grade UC [
3,
16].
NKX3.1, a prostate specific androgen regulated homeobox gene [
20], is expressed in the prostatic epithelium, rare ureteral and urothelial cells, normal testis, lobular carcinoma of the breast, and bronchial mucous glands [
21,
22]. Gelmann
et al. [
22] reported that all 40 observed cases of UC were negative for NKX3.1. In the current study, none of the 138 cases of UC was positive for NKX3.1. The sensitivities of NKX3.1 for PAC reported in previous studies were 92.1%, 89.5%, 87.4%, and 69.2% [
3,
21-
23]. This study also showed a comparable result, with 88.3% sensitivity of NKX3.1 for PAC.
The AMACR, localized predominantly in peroxisomal structures, plays a critical role in peroxisomal beta oxidation of branched chain fatty acid. Jiang
et al. [
24] demonstrated that both PAC and high-grade prostate intraepithelial neoplasia (HG-PIN) consistently revealed a significantly higher expression than normal epithelium. However, AMACR expression has repeatedly been demonstrated in HG-PIN and some benign mimickers of PAC. Moreover, Kunju
et al. [
6] reported that AMACR is expressed in 36% of UC cases. In our study, AMACR was expressed in 66.7% of 111 cases of PAC and 8.7% of 138 cases of UC. AMACR is less sensitive than other prostate markers for PAC and is of limited utility in resolving the difficult problems involving both the prostate and urinary bladder.
Although PSA, PSMA, PAP, P501s, and NKX3.1 are sensitive and specific markers for evaluating the prostatic origin of tumors, lack of staining was also detected for most markers, except PSA, in this study, at 16.2% for PSMA, 8.1% for PAP, 6.3% for P501s, and 11.7% for NKX3.1 of 111 PAC cases. Therefore, the lack of immunoreactivity of prostate markers in a poorly differentiated carcinoma does not exclude the possibility of a prostatic origin. In addition, false-positives were detected in UC in five of six established prostate markers in this study, ranging from 0.7% to 18.8%, suggesting that the immunohistochemical panel is necessary and useful to discriminate poorly differentiated high-grade carcinomas involving both the prostate and bladder.
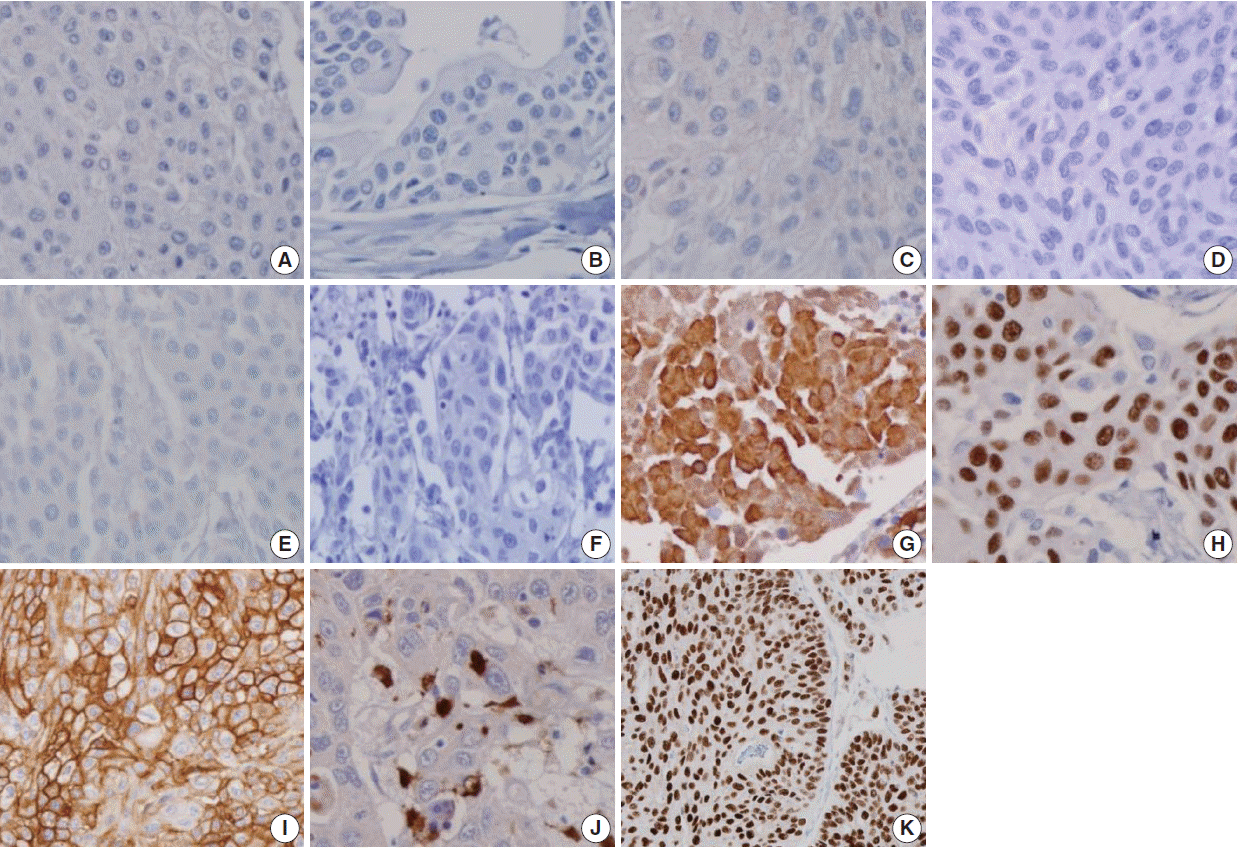
Many immunohistochemical stains have been investigated for UC, but no single marker has been found to be unequivocally diagnostic of urothelial origin. Thus, investigators have recommended a panel of markers to demonstrate the urothelial origin of tumor, such as CK34βE12, p63, thrombomodulin, S100P, and GATA3.
The monoclonal antibody CK34βE12, which reactive specifically against high-molecular-weight cytokeratins (CKs), including CK1, CK5, CK14, and CK20 [
2], is an extremely sensitive marker of urothelial lineage. It is reported to match the sensitivity of p63 and surpass that of uroplakin III and thrombomodulin [
3,
25]. Compared with previous studies showing sensitivities of 97.2%, 91.4%, and 65.2% for CK34βE12 in UC [
3,
6,
10], our study found 75.4% sensitivity in UC. It is worth noting that CK34βE12 can label squamous epithelia, including areas of squamous differentiation in recurrent PAC after therapy. Thus, Parwani
et al. [
26] argued that immunopositivity for CK34βE12 restricted to areas of squamous differentiation does not exclude the possibility of PAC.
p63, a homologue of the p53 tumor suppressor gene, encodes at least six different proteins with a wide range of biologic functions, including a role in urothelial differentiation [
2]. Immunostaining for p63 is typically present in more than 90% of the nuclei of the normal urothelia [
2]. Many UCs retain a pattern of p63 expression, but p63 expression may be partially lost in high-grade UC [
3,
27]. Although p63 sensitivity for UC in our study (73.9%) was lower than that of previous studies (82.9%–91.7%) [
3,
6], its specificity was 100% for UC.
Thrombomodulin, also designated CD141, is an endothelial cell associated cofactor for the thrombin-mediated activator of protein C [
2]. Previous studies have shown that thrombomodulin was immunostained in 48.8%–68.6% of UC [
3,
5], but our study found a slightly lower expression at 45.7%.
S100P is highly expressed in the urothelial epithelium [
28]. Higgins
et al. [
28] reported that the polyclonal antibody against S100P labeled 85% of UC and 3% of PAC, whereas the monoclonal antibody against S100P detected 77% of UC and 2% of PAC. Chuang
et al. [
3] also reported that the monoclonal S100P detected 51.4% of UC and 7.9% of PAC. In our study with a monoclonal antibody against S100P, 22.5% of UC and 3.6% of PAC were stained, which was less than in previous studies [
3,
28].
Although CK34βE12 and p63 have been reported to intermittently label PAC in a non-basal cell distribution, thrombomodulin has not been reported to show cross reactivity [
5,
6,
10]. We found that CK34βE12 and p63 immunostains were superior to thrombomodulin or S100P as differential markers of urothelial origin. Only a few scattered cells of PAC were labeled with CK34βE12 (1.8%) and S100P (3.6%), but no PAC was immunopositive for thrombomodulin or p63 in our study.
GATA3 is a member of a zinc finger transcription factor family that plays an important role in promoting and directing cell proliferation, differentiation, and development [
2]. GATA3 is a very sensitive marker for UC, and it is also highly specific in excluding high-grade PAC [
29]. Chang
et al. [
29] reported that none of the 38 high-grade PACs was positive for GATA3. In this study, the sensitivity of GATA3 was 0% in PAC and 84.8% in UC. Uroplakin III is considered the most specific marker for urothelial differentiation, but it has not received popularity due to the lack of uniform expression in UCs [
29]. Our study has some limitations because we did not include studies of Uroplakin III.
In conclusion, prostatic markers, including PSA, PSMA, PAP, and P501s, are very useful for distinguishing PAC from UC. Urothelial markers are less sensitive in identifying UC but rarely stain PAC. In the current study, we found that PSA is most sensitive prostatic marker for distinguishing PAC from UC cases with high sensitivity and negative predictive value. In addition, NKX3.1 is the most specific prostatic marker for distinguishing PAC from UC cases with high specificity and positive predictive value. p63 and thrombomodulin are the most specific urothelial markers for distinguishing UC from PAC cases with high specificities. GATA3 was positive in 117 of 137 cases of UCs and none of the 111 PACs was positive for GATA3. We found that the best combination of immunohistochemical markers for distinguishing PAC from UC is panels consisting of PSA, NKX3.1, p63, thrombomodulin, and GATA3. The optimal combination of immunohistochemical panels of prostatic and urothelial markers could improve the ability to establish the pathologic diagnosis of poorly differentiated high-grade carcinomas involving either the prostate or urinary bladder.
Go to :






 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download