1. Mercante G, Frasoldati A, Pedroni C, et al. Prognostic factors affecting neck lymph node recurrence and distant metastasis in papillary microcarcinoma of the thyroid: results of a study in 445 patients. Thyroid. 2009; 19:707–16.

2. Enewold L, Zhu K, Ron E, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980-2005. Cancer Epidemiol Biomarkers Prev. 2009; 18:784–91.

3. Park YJ, Kim YA, Lee YJ, et al. Papillary microcarcinoma in comparison with larger papillary thyroid carcinoma in BRAFV600E mutation, clinicopathological features, and immunohistochemical findings. Head Neck. 2010; 32:38–45.
4. Ito Y, Miyauchi A, Inoue H, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg. 2010; 34:28–35.

5. Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, Fujimoto Y. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg. 2010; 34:1222–31.

6. Mazzaferri EL. Managing small thyroid cancers. JAMA. 2006; 295:2179–82.

7. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–214.

8. Lee E, Jung W, Woo JS, et al. Tumor sprouting in papillary thyroid carcinoma is correlated with lymph node metastasis and recurrence. Korean J Pathol. 2014; 48:117–25.

9. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer-Verlag;2009. p. 87–96.
10. Mazeh H, Samet Y, Hochstein D, et al. Multifocality in well-differentiated thyroid carcinomas calls for total thyroidectomy. Am J Surg. 2011; 201:770–5.

11. Dietlein M, Luyken WA, Schicha H, Larena-Avellaneda A. Incidental multifocal papillary microcarcinomas of the thyroid: is subtotal thyroidectomy combined with radioiodine ablation enough? Nucl Med Commun. 2005; 26:3–8.

12. Rodríguez-Cuevas S, Labastida-Almendaro S, Cortés-Arroyo H, López-Garza J, Barroso-Bravo S. Multifactorial analysis of survival and recurrences in differentiated thyroid cancer: comparative evaluation of usefulness of AGES, MACIS, and risk group scores in Mexican population. J Exp Clin Cancer Res. 2002; 21:79–86.
13. Voutilainen PE, Siironen P, Franssila KO, Sivula A, Haapiainen RK, Haglund CH. AMES, MACIS and TNM prognostic classifications in papillary thyroid carcinoma. Anticancer Res. 2003; 23:4283–8.
14. Pyo JS, Sohn JH, Kang G, Kim DH, Yun J. Total surface area is useful for differentiating between aggressive and favorable multifocal papillary thyroid carcinomas. Yonsei Med J. 2015; 56:355–61.

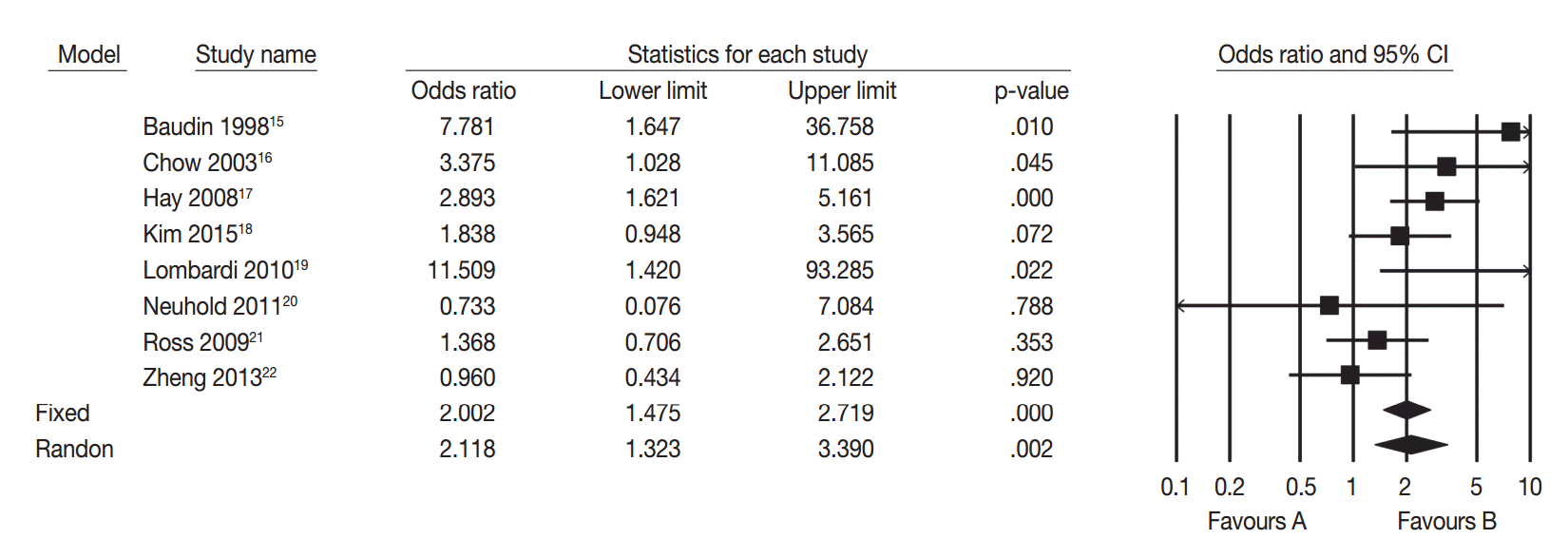
15. Baudin E, Travagli JP, Ropers J, et al. Microcarcinoma of the thyroid gland: the Gustave-Roussy Institute experience. Cancer. 1998; 83:553–9.
16. Chow SM, Law SC, Chan JK, Au SK, Yau S, Lau WH. Papillary microcarcinoma of the thyroid-Prognostic significance of lymph node metastasis and multifocality. Cancer. 2003; 98:31–40.

17. Hay ID, Hutchinson ME, Gonzalez-Losada T, et al. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008; 144:980–7.

18. Kim KJ, Kim SM, Lee YS, Chung WY, Chang HS, Park CS. Prognostic significance of tumor multifocality in papillary thyroid carcinoma and its relationship with primary tumor size: a retrospective study of 2,309 consecutive patients. Ann Surg Oncol. 2015; 22:125–31.

19. Lombardi CP, Bellantone R, De Crea C, et al. Papillary thyroid microcarcinoma: extrathyroidal extension, lymph node metastases, and risk factors for recurrence in a high prevalence of goiter area. World J Surg. 2010; 34:1214–21.

20. Neuhold N, Schultheis A, Hermann M, Krotla G, Koperek O, Birner P. Incidental papillary microcarcinoma of the thyroid: further evidence of a very low malignant potential: a retrospective clinicopathological study with up to 30 years of follow-up. Ann Surg Oncol. 2011; 18:3430–6.
21. Ross DS, Litofsky D, Ain KB, et al. Recurrence after treatment of micropapillary thyroid cancer. Thyroid. 2009; 19:1043–8.

22. Zheng X, Wei S, Han Y, et al. Papillary microcarcinoma of the thyroid: clinical characteristics and BRAFV600E mutational status of 977 cases. Ann Surg Oncol. 2013; 20:2266–73.

23. Haymart MR, Cayo M, Chen H. Papillary thyroid microcarcinomas: big decisions for a small tumor. Ann Surg Oncol. 2009; 16:3132–9.

24. Noguchi S, Yamashita H, Uchino S, Watanabe S. Papillary microcarcinoma. World J Surg. 2008; 32:747–53.

25. Mazzaferri EL. Managing thyroid microcarcinomas. Yonsei Med J. 2012; 53:1–14.

26. Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002; 87:1941–6.

27. Strate SM, Lee EL, Childers JH. Occult papillary carcinoma of the thyroid with distant metastases. Cancer. 1984; 54:1093–100.

28. Roh JL, Kim JM, Park CI. Central cervical nodal metastasis from papillary thyroid microcarcinoma: pattern and factors predictive of nodal metastasis. Ann Surg Oncol. 2008; 15:2482–6.

29. Cheema Y, Olson S, Elson D, Chen H. What is the biology and optimal treatment for papillary microcarcinoma of the thyroid? J Surg Res. 2006; 134:160–2.

30. Macedo FI, Mittal VK. Total thyroidectomy versus lobectomy as initial operation for small unilateral papillary thyroid carcinoma: a meta-analysis. Surg Oncol. 2015; 24:117–22.

31. Kuo SF, Lin SF, Chao TC, Hsueh C, Lin KJ, Lin JD. Prognosis of multifocal papillary thyroid carcinoma. Int J Endocrinol. 2013; 2013:809382.

32. Ito Y, Miyauchi A. Appropriate treatment for asymptomatic papillary microcarcinoma of the thyroid. Expert Opin Pharmacother. 2007; 8:3205–15.

33. Yu XM, Lloyd R, Chen H. Current treatment of papillary thyroid microcarcinoma. Adv Surg. 2012; 46:191–203.

34. Renshaw AA. Sensitivity of fine-needle aspiration for papillary carcinoma of the thyroid correlates with tumor size. Diagn Cytopathol. 2011; 39:471–4.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download