Mammary-type myofibroblastoma (MFB) is a rare, benign spindle cell neoplasm with myofibroblastic differentiation that is histologically identical to MFB of the breast and was first described by Wargotz
et al. in 1987 [
1]. The tumor occurs mainly in older men and postmenopausal women. The age at presentation ranges from 35 to 85 years [
2,
3]. Common locations are along the embryonic milkline with extension from the mid-axilla to the medial groin [
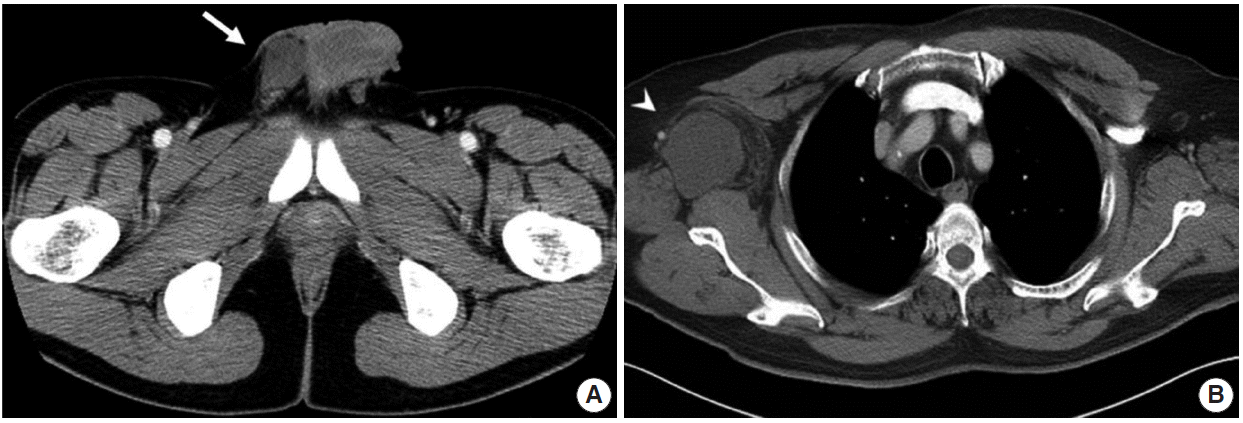
2]. The most common presentation is a painless, slowly growing mass [
2,
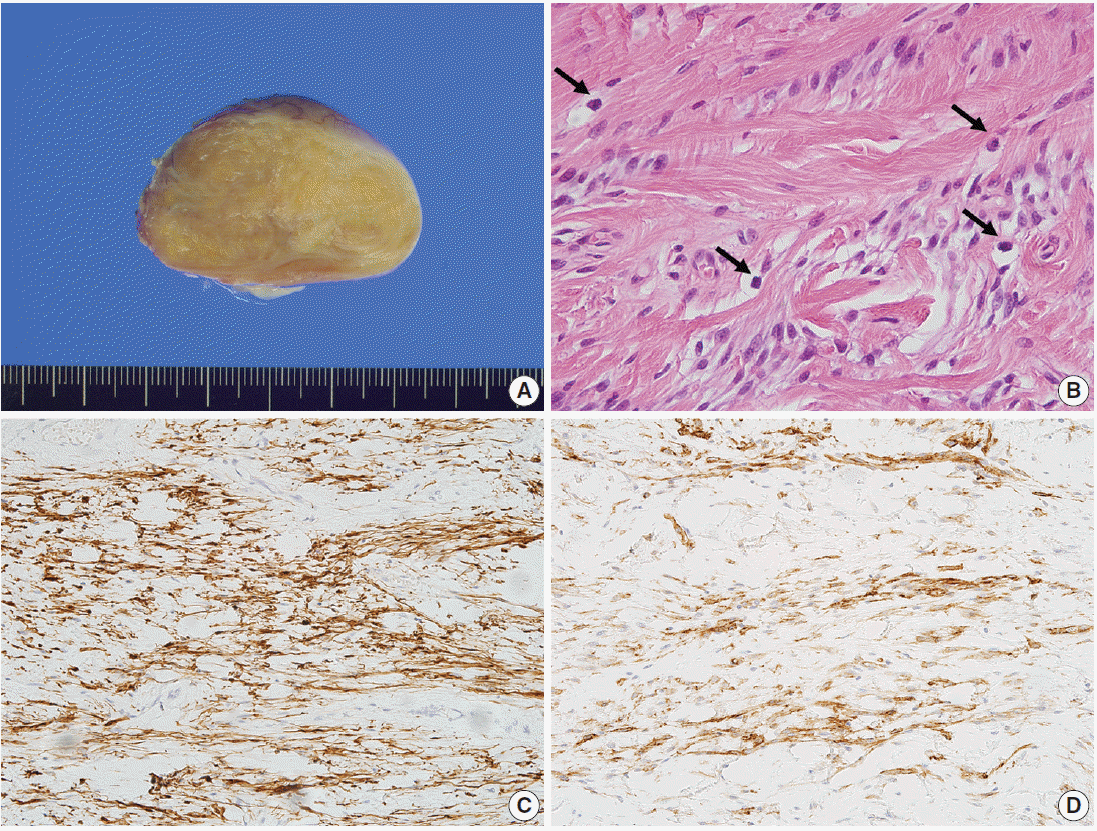
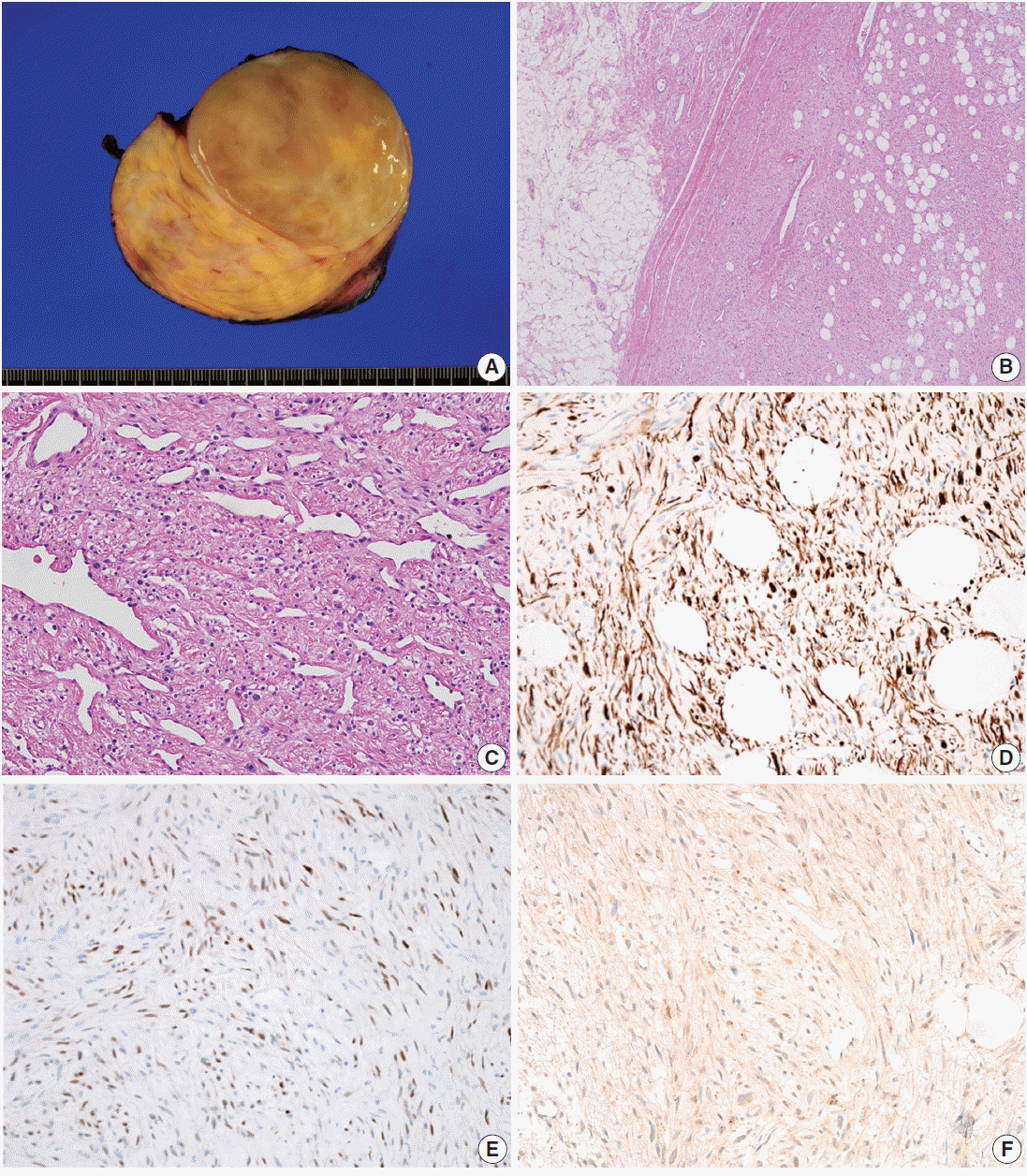
4]. The tumor is composed of bland spindle cells with myofibroblastic differentiation, prominent mast cells, fatty component, and hyalinized stroma. Recently, we experienced two cases of mammary-type MFB in the right scrotal sac of a 30-year-old man and in the right axilla of a 58-year-old man. In this report, we describe these two rare cases of mammary-type MFB.
DISCUSSION
Mammary-type MFB can occur along the embryonic milkline and accessory breast. Nevertheless, in many previously reported cases, the tumor did not contain any breast tissue. McMenamin and Fletcher [
2] described the possibility of ectopic breast in locations remote from the embryonic milkline. In addition, Millo
et al. [
5] suggested mesenchymal CD34-positive stem cells as an explanation for the hepatic location of mammary-type MFB.
MFB cells demonstrate immunopositivity for desmin and CD34 [
2,
6]. In addition, at least focal expression of androgen, estrogen, and progesterone receptors has been reported [
4]. Expression of smooth muscle actin is present in one-third of MFB cases [
7]. In the two cases presented here, the tumor cells showed immunopositivity for desmin and CD34 and immunonegativity for smooth muscle actin. The differential diagnoses of mammary-type MFB include other low-grade spindle cell neoplasms such as spindle cell lipoma, cellular angiofibroma, and solitary fibrous tumors (SFTs). Desmin, CD34, and estrogen receptors are helpful markers for differential diagnosis [
4,
6]. Recently, Howitt and Fletcher [
6] reported a large series of 143 cases of mammary-type MFB. They described complicated situations with mammary-type MFB, such as morphologic variation and CD34 and/or desmin negativity [
6]. Spindle cell lipoma and cellular angiofibroma show overlapping histologic and immunophenotypic features with mammary-type MFB and share similar genetic findings, with loss of 13q14 [
1,
2,
8,
9].
Spindle cell lipoma has similar histologic features to MFB such as bland spindle cells, abundant mast cells in the stroma, and admixed adipose tissue. The morphological differences between these two tumor types are subtle. The spindle cells of mammary-type MFB show a fascicular arrangement, whereas those of spindle cell lipoma show a more haphazard arrangement. CD34 expression is present in both tumors, whereas desmin immunopositivity is rarely present in spindle cell lipoma (<2%) [
2]. Maggiani
et al. [
9] explained these different histologic features and immunoprofiles as due to stromal precursor cells with a variable capacity to differentiate into fibroblastic/lipocytic or myofibroblastic cells. Cellular angiofibroma usually involves the groin/pelvic area [
2,
10]. Histologically, cellular angiofibroma is composed of short spindle cells arranged without any pattern and numerous small- to medium-sized thick-walled hyalinized vessels. Some tumors contain a variable amount of adipose tissue [
2,
10,
11]. Tumor cells can express CD34, but smooth muscle actin and desmin are usually negative [
10,
11].
Mammary-type MFB, spindle cell lipoma, and cellular angiofibroma share not only histologic features, but also similar genetic alterations. The
RB1 gene, as well as the
FOXO1 (forkhead box protein O1, previously known as FKHR, forkhead in rhabdomyosarcoma) gene, is located in the 13q14 tumor suppressor locus, and deletion of this region, which was originally identified in spindle cell lipoma, has been reported in several cases of mammary-type MFB and cellular angiofibroma [
9]. Maggiani
et al. [
9] described the loss of
RB/13q14 and
FKHR/13q14 loci in mammary-type MFB using fluorescence in situ hybridization (FISH) and suggested a genetic link with spindle cell lipoma, which has a characteristic loss of 13q and 16q. Hox
et al. [
8] reported a loss of
RB/13q14 loci in mammary-type MFB of the head and neck region. Magro
et al. [
12] described a deletion of
FOXO1, which is located on 13q14.11, both in mammary and vaginal MFB. In that study, five of seven cases of mammary-type MFB and three of five cases of vaginal MFB showed deletion of this locus. Hameed
et al. [
1] reported monosomy of chromosome 16 and loss of chromosome 13 in cellular angiofibroma. Flucke
et al. [
10] described the heterozygous loss of
RB1 in seven cases of cellular angiofibroma according to FISH. Magro [
13] suggested the term benign stromal/mesenchymal tumor with 13q14 deletion, as these three entities share the same genetic alteration, and a minority of cases of spindle cell lipoma and cellular angiofibroma showed histologic similarity to mammary-type MFB.
SFT is another possible differential diagnosis. The tumor is composed of spindle cells surrounding thick-walled, branching staghorn-like blood vessels. An admixed adipocytic component can be present [
2,
11]. In SFT, the spindle cells show a haphazard arrangement with intertwining thin collagen fibers, whereas those in mammary-type MFB show short fascicles with broad interrupting collagen bands. Oval myoid cells with abundant eosinophilic cytoplasm, which are distinguishing features of mammary-type MFB, are not observed in SFT. SFT commonly expresses CD34 (90%) and CD99 (70%), especially the fibrous form [
14]. Although SFT shares the histological and immunohistochemical characteristics of mammary-type MFB, the cytogenetics of SFT are different from those of mammary-type MFB. The absence of
RB/13q14 loss in SFTs is the key point for the differential diagnosis [
11,
15].
We report these cases due to the rare incidence and diagnostic utility of desmin immunoreactivity. We conclude that, if spindle cell lesions with myofibroblastic differentiation and admixed adipose tissue and mast cells in unusual locations are observed, the diagnosis of mammary-type MFB should be considered as a differential diagnosis, and desmin immunopositivity could be helpful for diagnosis.
Go to :





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download