MATERIALS AND METHODS
Table 1.
SFT, solitary fibrous tumor; HPC, hemangiopericytoma; Tx, therapy; F, female; HPC, hemangiopericytoma; GTR, gross total resection; RT, radiotherapy; DBD, death by disease; Rt., right; L/E, lower extremity; CT, chemotherapy; VIP, VP-16, ifosfamide, cisplatin; Lt., left; GKS, gamma knife surgery; M, male; STR, subtotal resection; EAC, external auditory canal; CPA, cerebellopontine angle; T-P-O, temporoparietooccipital lobe; SFT, solitary fibrous tumor; F-T, frontotemporal; NA, not applicable.
Table 2.
RESULTS
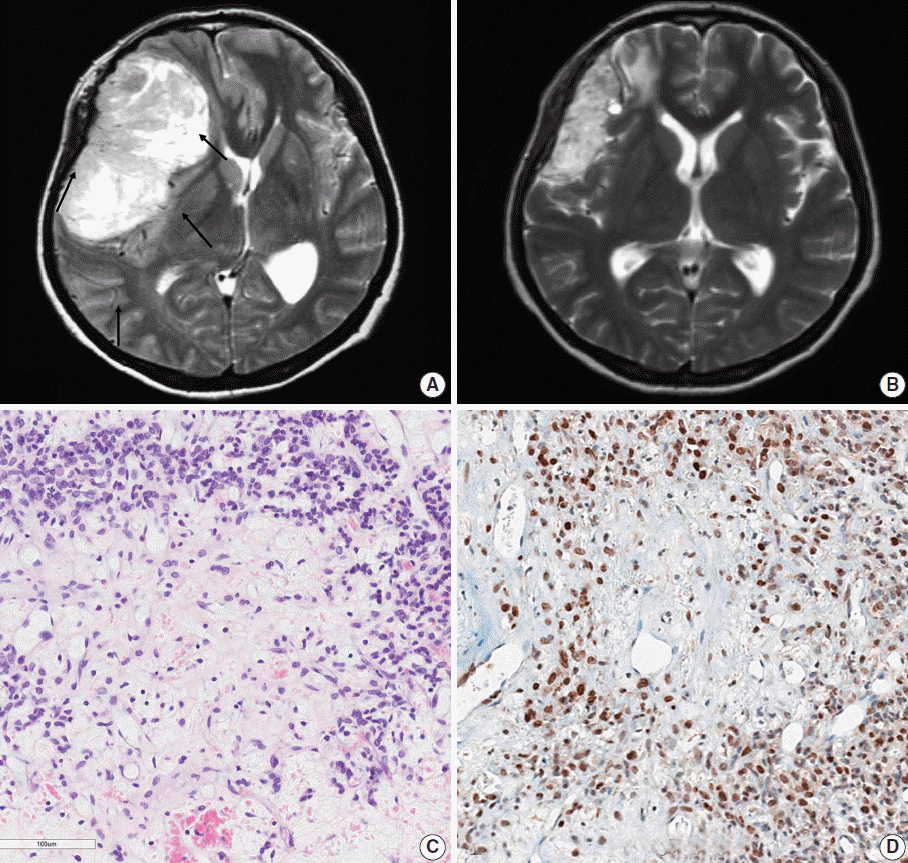 | Fig. 1.Representative brain magnetic resonance images and histopathology of the primary brain tumor from case 10. (A) Prior to radiation therapy. A large, solid and cystic mass is observed in the right frontoparietal lobe with strong enhancement. There was severe mass effect. Intratumoral vessels were identified. (B) After preoperative radiation therapy. The tumor decreased in the size after radiation therapy. (C) The tumor demonstrates low cellular and edematous areas with staghorn-shaped vasculature. The tumor cells may have been ablated by radiotherapy. (D) Aberrant STAT6 nuclear positivity is observed in the primary brain tumor. |
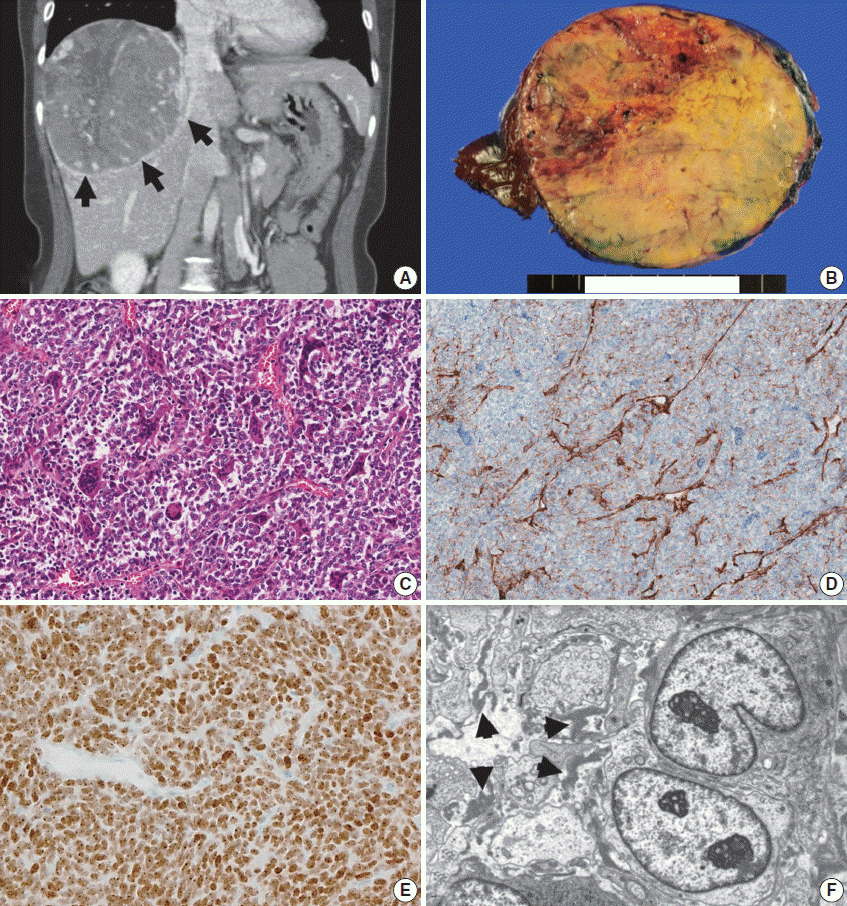 | Fig. 2.Computed tomography (CT) and pathology of a metastatic solitary fibrous tumor from case 10. (A) Liver CT scan showing a large, 12-cm soft tissue mass in the right lobe with prominent feeding vessels. (B) The cut surface of a hepatic tumor specimen showing a well-circumscribed, yellowish-tan-colored, solid tumor with multifocal necrosis, and hemorrhage with dilated vessels. (C) Microscopic examination of the liver mass reveals small, discohesive tumor cells with oval nuclei and fine chromatin. Staghorn-shaped hyalinized vessels and osteoclast-like multinucleated giant cells are identified. (D) Tumor cells are focally positive for CD34 on immunohistochemical staining. (E) Aberrant STAT6 nuclear positivity is observed in the metastatic tumor cells in the liver. (F) Ultrastructurally, the tumor is composed of sheets of oval to elongate cells around small capillaries. The tumor cells have nuclei with an oval or indented appearance, and fine, granular chromatin. Skein-like nuclei are also present. The individual tumor cells are surrounded by a thick, electron-dense, amorphous, external laminar material (arrowheads; uranyl acetate and lead citrate, × 5,000). |
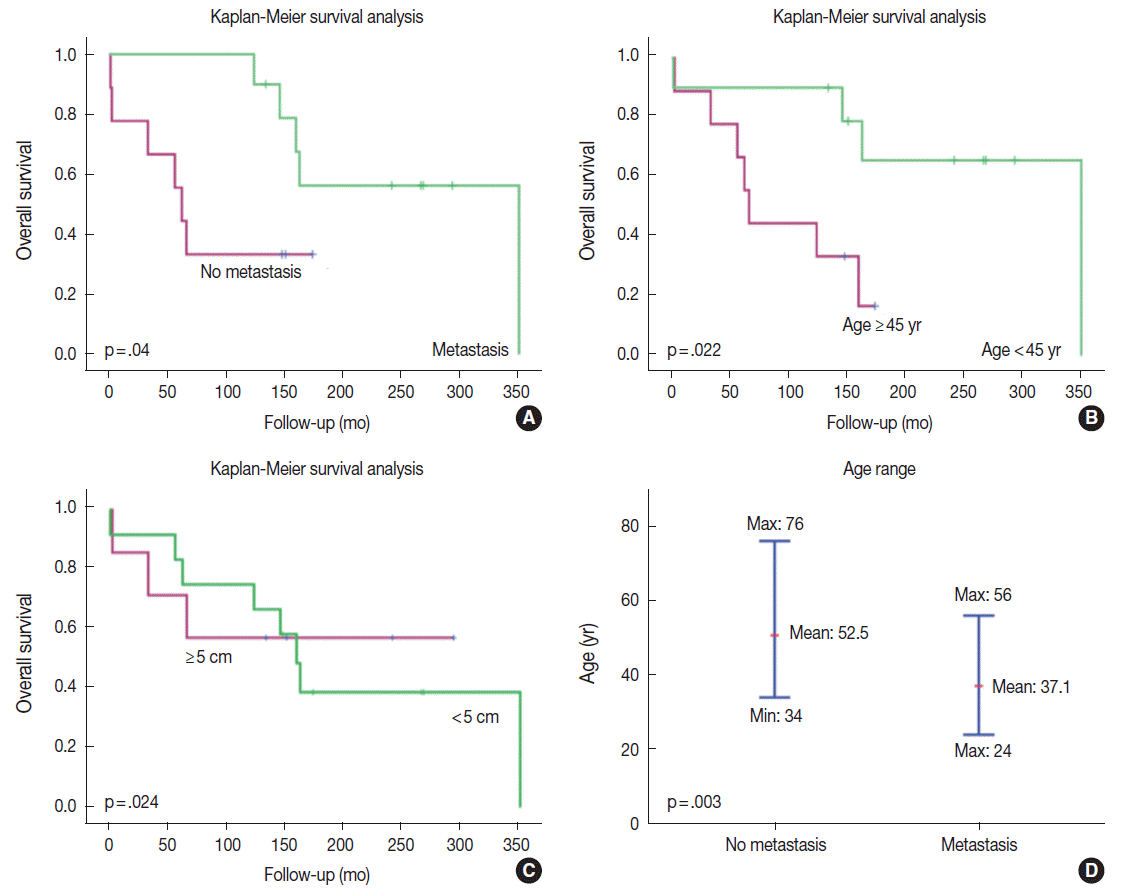 | Fig. 3.Kaplan-Meier survival curves of patients with solitary fibrous tumors according to the presence or absence of systemic metastases. (A) Paradoxically, the patients with systemic metastases had better prognosis than did those without metastasis. Metastases usually arose approximately 10 years after the initial tumor development. Therefore, only longer survivors developed systemic metastases. (B) Younger patients lived longer and had better survival. The age cut-off to demonstrate a meaningful survival difference is 45 years. (C) Patients with an initial tumor size > 5 cm have poorer survival than did those with smaller tumors. (D) The age of tumor onset is significantly younger in patients with systemic metastases than it is in patients without metastases. The median ages of onset in patients with and without systemic metastases are 37.1 years and 52.5 years, respectively. |
Table 3.
DISCUSSION
Table 4.
| Reference | Age (yr)/Sex | Diagnosis | Metastatic location | Time for metastasis (yr) |
|---|---|---|---|---|
| Ng et al. (2000) [14] | 55/F | SFT | Lung and neck | 9 |
| Someya et al. (2001) [15] | 42/F | HPC | Bone, lung, and liver | 12 |
| 37/F | HPC | Bone | 7 | |
| Dufour et al. (2001) [16] | 23/M | HPC | Bone | 12 |
| 22/F | HPC | Extracranial site | 13 | |
| 30/M | HPC | Extracranial site | 14 | |
| Ogawa et al. (2004) [13] | 44/F | SFT | Lung | 25 |
| Pistolesi et al. (2004) [7] | 42/F | HPC | Bone, lung and adrenal gland | 13 |
| Chang et al. (2004) [17] | 43/F | HPC | C2–C3 vertebrates, lung, liver and kidney | 5 |
| Metellus et al. (2007) [10] | 34/M | SFT | Systemic metastasis | 10.5 |
| Hayashi et al. (2009) [18] | 45/M | HPC | Extracranial site | NM |
| 30/M | HPC | Extracranial site | NM | |
| 47/F | HPC | Extracranial site | NM | |
| 35/M | HPC | Extracranial site | NM | |
| Ambrosini-Spaltro and Eusebi (2010) [11] | 51/F | HPC | Hip | 13 |
| Robinson et al. (2013) [20] | 40/M | Malignant SFT | Lung | NM |
| 33/F | Malignant SFT | Kidney | NM | |
| 29/M | Malignant SFT | Pancreas | NM | |
| 32/M | Malignant SFT | Small bowel | NM |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download