Abstract
Acute atherosis is unique vascular changes of the placenta associated with poor placentation. It is characterized by subendothelial lipid-filled foam cells, fibrinoid necrosis of the arterial wall, perivascular lymphocytic infiltration, and it is histologically similar to early-stage atherosclerosis. Acute atherosis is rare in normal pregnancies, but is frequently observed in non- transformed spiral arteries in abnormal pregnancies, such as preeclampsia, small for gestational age (SGA), fetal death, spontaneous preterm labor and preterm premature rupture of membranes. In preeclampsia, spiral arteries fail to develop physiologic transformation and retain thick walls and a narrow lumen. Failure of physiologic transformation of spiral arteries is believed to be the main cause of uteroplacental ischemia, which can lead to the production of anti-angiogenic factors and induce endothelial dysfunction and eventually predispose the pregnancy to preeclampsia. Acute atherosis is more frequently observed in the spiral arteries of the decidua of the placenta (parietalis or basalis) than in the decidual or myometrial segments of the placental bed. The presence and deeper location of acute atherosis is associated with poorer pregnancy outcomes, more severe disease, earlier onset of preeclampsia, and a greater frequency of SGA neonates in patients with preeclampsia. Moreover, the idea that the presence of acute atherosis in the placenta may increase the risk of future cardiovascular disease in women with a history of preeclampsia is of growing concern. Therefore, placental examination is crucial for retrospective investigation of pregnancy complications and outcomes, and accurate placental pathology based on universal diagnostic criteria in patients with abnormal pregnancies is essential for clinicopathologic correlation.
Acute atherosis was first described in 1945 by Hertig [1] and was named by Zeek and Assali [2]. Acute atherosis is unique vascular changes observed in non-transformed spiral arteries of the placenta. The histologic findings of acute atherosis, including fibrinoid necrosis, inflammatory cell infiltration of the vessel walls and collection of subendothelial lipid-laden macrophages [1-49], are similar to those of early-stage atherosclerosis of the coronary and other larger arteries, as well as allograft rejection [27,50]. Acute atherosis is rare in normal pregnancy [2,5,22,31,42], but is frequently observed in abnormal pregnancies, such as preeclampsia, small for gestational age (SGA), fetal death, spontaneous preterm labor and preterm premature rupture of membranes (PROM) [2,4,5,7-13,16-19,21,23,24,26-31,40-42,44-48,51]. The etiology and pathogenesis of preeclampsia is complicated and remains unclear, despite its role as one of the leading causes of maternal and neonatal morbidity and mortality in pregnancy. Failure of physiologic remodeling of the spiral arteries is a main cause of preeclampsia, and these arteries are prone to develop acute atherosis [52]. Moreover, accumulating evidence suggests that women with a history of preeclampsia exhibit a high prevalence of major cardiovascular risk factors [53-57]. This gives rise to the question of whether acute atherosis in the placenta is related to possible future cardiovascular disease in the mother.
This review describes (1) spiral artery changes in normal and abnormal placentation during pregnancy, (2) histologic findings of acute atherosis, (3) acute atherosis frequency in normal and abnormal pregnancies, (4) placental lesions associated with acute atherosis, (5) possible pathogenic mechanisms of acute atherosis, and (6) clinical implications of acute atherosis.
Normal physiologic transformation of the uterine spiral arteries during early pregnancy is considered a foundation of successful pregnancy [58]. The spiral arteries are normally transformed into large dilated vessels, with dramatic structural changes to the vessel wall. The key findings of normal transformation of the spiral arteries are (1) dilation of the lumen, (2) trophoblast invasion into the vessel wall, and (3) replacement of the muscular and elastic tissue of the arterial wall by a thick fibrinoid material (Fig. 1A) [58,59]. These changes maximize the delivery of maternal blood to the intervillous space of the placenta, so that a sufficient blood supply through transformed spiral arteries enables the transfer of enough nutrition and oxygen from the mother to the fetus. Maternal blood from dilated spiral arteries meets fetal blood in the intervillous space, while the intervillous space drains blood back to the utero-placental veins [60]. For successful placentation, trophoblast invasion from the maternal-fetal interface to the myometrium through the decidua during the first 3 months of pregnancy is critical.
Poor placentation is defined as the failure of physiologic transformation of spiral arteries and appears to arise from an inadequate or shallow trophoblast invasion [8,58,61]. Poor placentation is also a leading cause of preeclampsia and other abnormal pregnancies, such as spontaneous abortion, SGA, preterm labor and preterm PROM [7,9,62-65]. In preeclampsia, spiral arteries of the myometrial segment of the uterus fail to achieve physiologic transformation and retain thick walls and a narrow lumen; this is believed to be the main cause of uteroplacental ischemia (Fig. 1B) [7,9]. Uteroplacental ischemia can lead to the production of anti-angiogenic factors [66-68], such as soluble fms-like tyrosine kinase 1 [64,69], and endoglin [70,71], which can induce endothelial dysfunction and eventually predispose the pregnancy to preeclampsia. Spiral arteries that fail to achieve physiologic transformation are prone to develop acute atherosis [72-79].
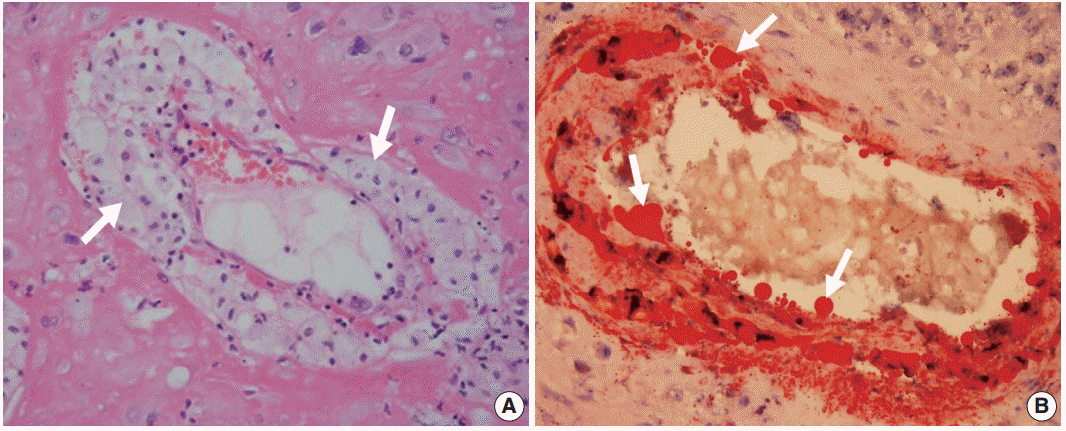
Histologic findings of acute atherosis consist of the presence of fibrinoid necrosis of the artery wall, a subendothelial collection of lipid-laden macrophages, and vascular or perivascular lymphocytic infiltration in non-transformed uterine spiral arteries (Fig. 2A) [1,11]. Lipids in the spiral arteries with acute atherosis are stained red with oil-red O (Fig. 2B).
Acute atherosis was more frequently observed in the spiral arteries of the decidua (parietalis or basalis) of the placenta than in the decidual or myometrial segments of the placental bed. The depth of acute atherosis is associated with the severity of preeclampsia. Briefly, the presence of atherosis in the myometrial segment is associated with a severe form and an earlier onset of preeclampsia than those without this lesion in the myometrial segment [51,80].
Acute atherosis used to be considered a characteristic finding of spiral arteries of patients with preeclampsia and has been reported to occur in 5% to 40% of patients with preeclampsia [5,7,8,10-13,16-19,23,24,26-31,40,42,45,46]. The variable frequency of acute atherosis may be explained by the following reasons: (1) variation in the number of tissue sections taken (size of sample), (2) location of tissue sections, (3) variation in tissue staining methods (hematoxylin and eosin [H&E] staining only or additional immunohistochemical staining) for the diagnosis of acute atherosis, and (4) differences in the pathologist’s diagnostic skill. In a previous study with over 14,000 placenta samples using only H&E staining for the diagnosis of acute atherosis and taking an average of two sections from each placenta based on routine histology laboratory tasks [51], the prevalence of acute atherosis in uncomplicated pregnancies was 0.4% and the frequency of acute atherosis varied based on the specific obstetrical syndrome: preeclampsia, 10%; fetal death, 9%; mid-trimester spontaneous abortion, 2.5%; SGA neonates without preeclampsia, 1.7%; and spontaneous preterm labor, 1.2%. Acute atherosis is associated with more severe disease, earlier onset of preeclampsia, and a greater frequency of SGA neonates in patients with preeclampsia [51,80].
A previous study found that acute atherosis is associated with an increased risk of placental lesions consistent with maternal underperfusion, fetal vascular thrombo-occlusive disease and chronic chorioamnionitis, but not with other chronic inflammatory lesions [81].
The correlation between acute atherosis and chronic chorioamnionitis indicates the presence of circulating maternal T cells and adaptive immune response may also play a role in the genesis of acute atherosis [73,77,78,82-91].
In chronic chorioamnionitis, maternal T cells infiltrating the chorion laeve cause trophoblast apoptosis, which resembles allograft rejection [92]. Chronic chorioamnionitis is also associated with anti-fetal HLA maternal sensitization [93] and complement deposition in the umbilical vein endothelium [94], which has been associated with a novel form of fetal systemic inflammatory response characterized by the over-expression of T-cell chemokines such as CXCL10 [95].
Higher concentrations of CXCL9, CXCL10, and CXCL11 have been found in mothers with chronic placental inflammation compared to those without [92]. Similarly, T lymphocytes have been detected in the early stages of atheroma formation [96]. Moreover, differential expression of three interferon (IFN) gammainducible CXC chemokines, IFN-inducible protein 10 (CXCL10 or IP-10), monokine induced by IFN-y (CXCL9 or Mig) and IFN-inducible T-cell α chemoattractant (CXCL11 or I-TAC) were found in atherosclerosis [96].
Multiple factors including excessive decidual inflammation [42,47,48,97], immune dysregulation at the maternal-fetal interface [27] and immunological mismatch between the mother and fetus [27] have been proposed as causes or initiators of acute atherosis.
Recently, Staff et al. [48] suggested four serial mechanisms for the development of acute atherosis, with excessive decidual inflammation as the final common pathway: (1) shear flow stress caused by abnormal blood flow in inadequately remodeled spiral arteries, (2) decidual inflammation, including maternal alloreactivity to feto-paternal HLA-C or minor histocompatibility antigens, (3) background (systemic) maternal inflammatory stress secondary to the changes induced by pregnancy and preeclampsia, and (4) maternal genetic predisposition (for example, polymorphism in regulator of G protein signaling 2).
Elevation of signs of intravascular inflammation have been reported in both normal [98-101] and abnormal pregnancies, such as spontaneous preterm labor with intact membranes [102-108], preterm PROM [109-114], preeclampsia [115-144], SGA [119,134,136,138,145-154], and pyelonephritis [99,155-157]. Since chronic vascular inflammation is one of the main causes of atherosclerosis and acute atherosis, the possibility of activation of cholesterol crystal-induced inflammasome in macrophages [158] should be investigated as an important link between cholesterol metabolism and acute atherosis.
In atherosclerosis, medium-sized and large arteries fueled by lipids, as well as the deposition of excess cholesterol in the blood stream, initiate atherosclerosis [159]. Similarly, acute atherosis in non-transformed uterine spiral arteries also show variable amounts of lipid deposition in the wall of spiral arteries, and it is stained red with oil-red O staining (Fig. 2B). Moreover, serum triglycerides are about 50% higher in preeclamptic women than in normal pregnant women. However, there are no differences in other lipid profiles, including total cholesterol and high-density lipoprotein [51,160-162]. Whether there are differences in low-density lipoprotein remains controversial [143-145,163].
Accumulating evidence suggests that women with a history of preeclampsia show a high prevalence of major cardiovascular risk factors [53-57]. Similarities between preeclampsia and atherosclerosis, as well as between acute atherosis of the spiral arteries and coronary atherosclerosis, have been observed. Intravascular inflammation [98,118,120,164,165], changes in lipid metabolism [162,166-168], and macrophage infiltration of the intima and media are seen in both acute atherosis and atherosclerosis [27,169,170]. Therefore, hyperlipidemia and abnormal lipid metabolism combined with intravascular inflammation can defect endothelial cell function and may lead to atherosclerosis in non-pregnant women who have a past history of preeclampsia [72-79]. We recommend that women who have a past history of preeclampsia be considered at high risk for cardiovascular disease and recommend implementation of regular screenings and prevention programs.
The presence and deeper location of acute atherosis is associated with worse pregnancy outcomes, more severe disease, earlier onset, and a greater frequency of SGA neonates in patients with preeclampsia. Moreover, the idea that acute atherosis in the placenta may increase the risk of future cardiovascular disease in women with a history of preeclampsia is of growing concern. Therefore, placental examination is crucial for investigation of pregnancy complications and outcomes, and accurate placental pathology based on universal diagnostic criteria in patients with abnormal pregnancies is essential for clinicopathologic correlation.
ACKNOWLEDGMENTS
The authors are very grateful to Professor Je G. Chi, who passed away during the winter of 2014. He was a great mentor and friend to all of us. This work is dedicated to his memory.
REFERENCES
1. Hertig AT. Vascular pathology in the hypertensive albuminuric toxemias of pregnancy. Clinics. 1945; 4:602–14.
2. Zeek PM, Assali NS. Vascular changes in the decidua associated with eclamptogenic toxemia of pregnancy. Am J Clin Pathol. 1950; 20:1099–109.

3. Hanssens M, Pijnenborg R, Keirse MJ, Vercruysse L, Verbist L, Van Assche FA. Renin-like immunoreactivity in uterus and placenta from normotensive and hypertensive pregnancies. Eur J Obstet Gynecol Reprod Biol. 1998; 81:177–84.

4. Sexton LI, Hertig AT, Reid DE, Kellogg FS, Patterson WA. Premature separation of the normally implanted placenta; a clinicopathological study of 476 cases. Am J Obstet Gynecol. 1950; 59:13–24.
5. Maqueo M, Chavezazuela J, Dosaldelavega M. Placental pathology in eclampsia and preeclampsia. Obstet Gynecol. 1964; 24:350–6.

6. Driscoll SG. The pathology of pregnancy complicated by diabetes mellitus. Med Clin N Am. 1965; 49:1053–67.

7. Robertson WB, Brosens I, Dixon HG. The pathological response of the vessels of the placental bed to hypertensive pregnancy. J Pathol Bacteriol. 1967; 93:581–92.

8. Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972; 1:177–91.
9. Brosens I, Renaer M. On the pathogenesis of placental infarcts in pre-eclampsia. J Obstet Gynaecol Br Commonw. 1972; 79:794–9.

10. Emmrich P, Birke R, Gödel E. Morphology of myometrial and decidual arteries in normal pregnancy, in toxemia of pregnancy, and in maternal diabetes (author’s transl). Pathol Microbiol (Basel). 1975; 43:38–61.
11. De Wolf F, Robertson WB, Brosens I. The ultrastructure of acute atherosis in hypertensive pregnancy. Am J Obstet Gynecol. 1975; 123:164–74.

12. Robertson WB, Brosens I, Dixon G. Uteroplacental vascular pathology. Eur J Obstet Gynecol Reprod Biol. 1975; 5:47–65.

13. Robertson WB, Brosens I, Dixon G. Maternal uterine vascular lesions in the hypertensive complications of pregnancy. Perspect Nephrol Hypertens. 1976; 5:115–27.
14. De Wolf F, Brosens I, Renaer M. Fetal growth retardation and the maternal arterial supply of the human placenta in the absence of sustained hypertension. Br J Obstet Gynaecol. 1980; 87:678–85.
15. Abramowsky CR, Vegas ME, Swinehart G, Gyves MT. Decidual vasculopathy of the placenta in lupus erythematosus. N Engl J Med. 1980; 303:668–72.

16. Kitzmiller JL, Watt N, Driscoll SG. Decidual arteriopathy in hypertension and diabetes in pregnancy: immunofluorescent studies. Am J Obstet Gynecol. 1981; 141:773–9.

17. Sheppard BL, Bonnar J. An ultrastructural study of utero-placental spiral arteries in hypertensive and normotensive pregnancy and fetal growth retardation. Br J Obstet Gynaecol. 1981; 88:695–705.

18. De Wolf F, Carreras LO, Moerman P, Vermylen J, Van Assche A, Renaer M. Decidual vasculopathy and extensive placental infarction in a patient with repeated thromboembolic accidents, recurrent fetal loss, and a lupus anticoagulant. Am J Obstet Gynecol. 1982; 142:829–34.

19. Hustin J, Foidart JM, Lambotte R. Maternal vascular lesions in preeclampsia and intrauterine growth retardation: light microscopy and immunofluorescence. Placenta. 1983; 4 Spec No:489–98.
20. Althabe O, Labarrere C, Telenta M. Maternal vascular lesions in placentae of small-for-gestational-age infants. Placenta. 1985; 6:265–76.

21. Labarrere C, Alonso J, Manni J, Domenichini E, Althabe O. Immunohistochemical findings in acute atherosis associated with intrauterine growth retardation. Am J Reprod Immunol Microbiol. 1985; 7:149–55.

22. Labarrere C, Althabe O. Chronic villitis of unknown etiology and maternal arterial lesions in preeclamptic pregnancies. Eur J Obstet Gynecol Reprod Biol. 1985; 20:1–11.

23. Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986; 93:1049–59.

24. McFadyen IR, Price AB, Geirsson RT. The relation of birthweight to histological appearances in vessels of the placental bed. Br J Obstet Gynaecol. 1986; 93:476–81.

25. Labarrere CA, Catoggio LJ, Mullen EG, Althabe OH. Placental lesions in maternal autoimmune diseases. Am J Reprod Immunol Microbiol. 1986; 12:78–86.

26. Khong TY, Pearce JM, Robertson WB. Acute atherosis in preeclampsia: maternal determinants and fetal outcome in the presence of the lesion. Am J Obstet Gynecol. 1987; 157:360–3.

27. Labarrere CA. Acute atherosis: a histopathological hallmark of immune aggression? Placenta. 1988; 9:95–108.
28. Frusca T, Morassi L, Pecorelli S, Grigolato P, Gastaldi A. Histological features of uteroplacental vessels in normal and hypertensive patients in relation to birthweight. Br J Obstet Gynaecol. 1989; 96:835–9.

29. Khong TY. Acute atherosis in pregnancies complicated by hypertension, small-for-gestational-age infants, and diabetes mellitus. Arch Pathol Lab Med. 1991; 115:722–5.
30. Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1994; 101:669–74.

31. Meekins JW, Pijnenborg R, Hanssens M, van Assche A, McFadyen IR. Immunohistochemical detection of lipoprotein(a) in the wall of placental bed spiral arteries in normal and severe preeclamptic pregnancies. Placenta. 1994; 15:511–24.

32. Salafia CM, Parke AL. Placental pathology in systemic lupus erythematosus and phospholipid antibody syndrome. Rheum Dis Clin North Am. 1997; 23:85–97.

33. Brosens I, Dixon HG, Robertson WB. Fetal growth retardation and the arteries of the placental bed. Br J Obstet Gynaecol. 1977; 84:656–63.

34. Gerretsen G, Huisjes HJ, Elema JD. Morphological changes of the spiral arteries in the placental bed in relation to pre-eclampsia and fetal growth retardation. Br J Obstet Gynaecol. 1981; 88:876–81.
35. Nayar R, Lage JM. Placental changes in a first trimester missed abortion in maternal systemic lupus erythematosus with antiphospholipid syndrome; a case report and review of the literature. Hum Pathol. 1996; 27:201–6.

36. Hayati AR, Azizah A, Wahidah A. Incidence of acute atherosis in complete molar pregnancy. Malays J Pathol. 1998; 20:113–4.
37. Khong TY, Hague WM. The placenta in maternal hyperhomocysteinaemia. Br J Obstet Gynaecol. 1999; 106:273–8.

38. Ogishima D, Matsumoto T, Nakamura Y, Yoshida K, Kuwabara Y. Placental pathology in systemic lupus erythematosus with antiphospholipid antibodies. Pathol Int. 2000; 50:224–9.

39. Sebire NJ, Rees H, Paradinas F, et al. Extravillus endovascular implantation site trophoblast invasion is abnormal in complete versus partial molar pregnancies. Placenta. 2001; 22:725–8.

40. Moldenhauer JS, Stanek J, Warshak C, Khoury J, Sibai B. The frequency and severity of placental findings in women with preeclampsia are gestational age dependent. Am J Obstet Gynecol. 2003; 189:1173–7.

41. Faye-Petersen O, Heller DS, Joshi VV. Handbook of placental pathology. 2nd ed. London: Taylor & Francis;2006. p. 53–78.
42. Harsem NK, Roald B, Braekke K, Staff AC. Acute atherosis in decidual tissue: not associated with systemic oxidative stress in preeclampsia. Placenta. 2007; 28:958–64.

43. Fox H, Sebire NJ. Pathology of theplacenta. 3rd ed. Philadelphia: Saunders-Elsevier;2007. p. 147–86.
44. Brosens I, Khong TY. Defective spiral artery remodeling. In : Pijnenborg R, Brosens I, Romero R, editors. Placental bed disorders. Cambridge: Cambridge University Press;2010. p. 11.
45. Staff AC, Dechend R, Pijnenborg R. Learning from the placenta: acute atherosis and vascular remodeling in preeclampsia-novel aspects for atherosclerosis and future cardiovascular health. Hypertension. 2010; 56:1026–34.
46. Stevens DU, Al-Nasiry S, Bulten J, Spaanderman ME. Decidual vasculopathy in preeclampsia: lesion characteristics relate to disease severity and perinatal outcome. Placenta. 2013; 34:805–9.
47. Staff AC, Dechend R, Redman CW. Review: preeclampsia, acute atherosis of the spiral arteries and future cardiovascular disease: two new hypotheses. Placenta. 2013; 34 Suppl:S73–8.

48. Staff AC, Johnsen GM, Dechend R, Redman CW. Preeclampsia and uteroplacental acute atherosis: immune and inflammatory factors. J Reprod Immunol. 2014; 101-102:120–6.

49. Staff AC, Redman CW. IFPA Award in Placentology Lecture: preeclampsia, the decidual battleground and future maternal cardiovascular disease. Placenta. 2014; 35 Suppl:S26–31.

50. Mitchell RN. Graft vascular disease: immune response meets the vessel wall. Annu Rev Pathol. 2009; 4:19–47.

51. Kim YM, Chaemsaithong P, Romero R, et al. The frequency of acute atherosis in normal pregnancy and preterm labor, preeclampsia, small-for-gestational age, fetal death and midtrimester spontaneous abortion. J Matern Fetal Neonatal Med. 2015; 28:2001–9.

52. Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006; 27:939–58.

53. van Rijn BB, Nijdam ME, Bruinse HW, et al. Cardiovascular disease risk factors in women with a history of early-onset preeclampsia. Obstet Gynecol. 2013; 121:1040–8.

54. Veerbeek JH, Hermes W, Breimer AY, et al. Cardiovascular disease risk factors after early-onset preeclampsia, late-onset preeclampsia, and pregnancy-induced hypertension. Hypertension. 2015; 65:600–6.

55. Sattar N, Ramsay J, Crawford L, Cheyne H, Greer IA. Classic and novel risk factor parameters in women with a history of preeclampsia. Hypertension. 2003; 42:39–42.

56. Berends AL, de Groot CJ, Sijbrands EJ, et al. Shared constitutional risks for maternal vascular-related pregnancy complications and future cardiovascular disease. Hypertension. 2008; 51:1034–41.

57. van Rijn BB, Veerbeek JH, Scholtens LC, et al. C-reactive protein and fibrinogen levels as determinants of recurrent preeclampsia: a prospective cohort study. J Hypertens. 2014; 32:408–14.
58. Brosens I, Robertson WB, Dixon HG. The physiological response of the vessels of the placental bed to normal pregnancy. J Pathol Bacteriol. 1967; 93:569–79.

59. Espinoza J, Romero R, Kim YM, et al. Normal and abnormal transformation of the spiral arteries during pregnancy. J Perinat Med. 2006; 34:447–58.

60. Freese UE. The uteroplacental vascular relationship in the human. Am J Obstet Gynecol. 1968; 101:8–16.

61. Brosens IA. Morphological changes in the utero-placental bed in pregnancy hypertension. Clin Obstet Gynaecol. 1977; 4:573–93.

62. Kim YM, Chaiworapongsa T, Gomez R, et al. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol. 2002; 187:1137–42.

63. Kim YM, Bujold E, Chaiworapongsa T, et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003; 189:1063–9.

64. Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005; 308:1592–4.

65. Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010; 376:631–44.

66. Makris A, Thornton C, Thompson J, et al. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int. 2007; 71:977–84.

67. Bujold E, Romero R, Chaiworapongsa T, et al. Evidence supporting that the excess of the sVEGFR-1 concentration in maternal plasma in preeclampsia has a uterine origin. J Matern Fetal Neonatal Med. 2005; 18:9–16.

68. Kusanovic JP, Romero R, Chaiworapongsa T, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009; 22:1021–38.

69. Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fmslike tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003; 111:649–58.

70. Lain KY, Roberts JM. Contemporary concepts of the pathogenesis and management of preeclampsia. JAMA. 2002; 287:3183–6.

71. Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006; 12:642–9.

74. Young JL, Libby P, Schönbeck U. Cytokines in the pathogenesis of atherosclerosis. Thromb Haemost. 2002; 88:554–67.

75. Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006; 6:508–19.

76. Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr Rev. 2007; 65(12 Pt 2):S140–6.

77. Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009; 6:399–409.

78. Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol. 2009; 27:165–97.
79. Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010; 74:213–20.
80. Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011; 204:193–201.

81. Kim YM, Chaemsaithong P, Romero R, et al. Placental lesions associated with acute atherosis. J Matern Fetal Neonatal Med. 2015; 28:1554–62.

82. Libby P. Atherosclerosis: disease biology affecting the coronary vasculature. Am J Cardiol. 2006; 98:3Q–9Q.

83. Libby P, Ridker PM, Hansson GK; Leducq Transatantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009; 54:2129–38.
84. Packard RR, Lichtman AH, Libby P. Innate and adaptive immunity in atherosclerosis. Semin Immunopathol. 2009; 31:5–22.

85. Andersson J, Libby P, Hansson GK. Adaptive immunity and atherosclerosis. Clin Immunol. 2010; 134:33–46.

86. Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011; 473:317–25.

87. Legein B, Temmerman L, Biessen EA, Lutgens E. Inflammation and immune system interactions in atherosclerosis. Cell Mol Life Sci. 2013; 70:3847–69.

88. Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013; 38:1092–104.

89. Rosenfeld ME. Inflammation and atherosclerosis: direct versus indirect mechanisms. Curr Opin Pharmacol. 2013; 13:154–60.

91. Ait-Oufella H, Sage AP, Mallat Z, Tedgui A. Adaptive (T and B cells) immunity and control by dendritic cells in atherosclerosis. Circ Res. 2014; 114:1640–60.

92. Kim CJ, Romero R, Kusanovic JP, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol. 2010; 23:1000–11.

93. Lee J, Romero R, Xu Y, et al. Maternal HLA panel-reactive antibodies in early gestation positively correlate with chronic chorioamnionitis: evidence in support of the chronic nature of maternal anti-fetal rejection. Am J Reprod Immunol. 2011; 66:510–26.

94. Lee J, Romero R, Xu Y, et al. A signature of maternal anti-fetal rejection in spontaneous preterm birth: chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PLoS One. 2011; 6:e16806.

95. Lee J, Kim JS, Park JW, et al. Chronic chorioamnionitis is the most common placental lesion in late preterm birth. Placenta. 2013; 34:681–9.

96. Mach F, Sauty A, Iarossi AS, et al. Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells. J Clin Invest. 1999; 104:1041–50.

97. Pijnenborg R, McLaughlin PJ, Vercruysse L, et al. Immunolocalization of tumour necrosis factor-alpha (TNF-alpha) in the placental bed of normotensive and hypertensive human pregnancies. Placenta. 1998; 19:231–9.
98. Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998; 179:80–6.

99. Naccasha N, Gervasi MT, Chaiworapongsa T, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol. 2001; 185:1118–23.

101. Watts DH, Krohn MA, Wener MH, Eschenbach DA. C-reactive protein in normal pregnancy. Obstet Gynecol. 1991; 77:176–80.

102. Gervasi MT, Chaiworapongsa T, Naccasha N, et al. Phenotypic and metabolic characteristics of maternal monocytes and granulocytes in preterm labor with intact membranes. Am J Obstet Gynecol. 2001; 185:1124–9.

103. Pitiphat W, Gillman MW, Joshipura KJ, Williams PL, Douglass CW, Rich-Edwards JW. Plasma C-reactive protein in early pregnancy and preterm delivery. Am J Epidemiol. 2005; 162:1108–13.

104. Gotsch F, Romero R, Kusanovic JP, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med. 2008; 21:529–47.

105. Kim MA, Lee BS, Park YW, Seo K. Serum markers for prediction of spontaneous preterm delivery in preterm labour. Eur J Clin Invest. 2011; 41:773–80.

106. Laudanski P, Raba G, Kuc P, Lemancewicz A, Kisielewski R, Laudanski T. Assessment of the selected biochemical markers in predicting preterm labour. J Matern Fetal Neonatal Med. 2012; 25:2696–9.

107. Cruciani L, Romero R, Vaisbuch E, et al. Pentraxin 3 in maternal circulation: an association with preterm labor and preterm PROM, but not with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2010; 23:1097–105.

108. Stampalija T, Chaiworapongsa T, Romero R, et al. Soluble ST2, a modulator of the inflammatory response, in preterm and term labor. J Matern Fetal Neonatal Med. 2014; 27:111–21.

109. Ismail MA, Zinaman MJ, Lowensohn RI, Moawad AH. The significance of C-reactive protein levels in women with premature rupture of membranes. Am J Obstet Gynecol. 1985; 151:541–4.

110. Yoon BH, Jun JK, Park KH, Syn HC, Gomez R, Romero R. Serum C-reactive protein, white blood cell count, and amniotic fluid white blood cell count in women with preterm premature rupture of membranes. Obstet Gynecol. 1996; 88:1034–40.
111. Gervasi MT, Chaiworapongsa T, Naccasha N, et al. Maternal intravascular inflammation in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2002; 11:171–5.

112. Loukovaara MJ, Alfthan HV, Kurki MT, Hiilesmaa VK, Andersson SH. Serum highly sensitive C-reactive protein in preterm premature rupture of membranes. Eur J Obstet Gynecol Reprod Biol. 2003; 110:26–8.

113. Moghaddam Banaem L, Mohamadi B, Asghari Jaafarabadi M, Aliyan Moghadam N. Maternal serum C-reactive protein in early pregnancy and occurrence of preterm premature rupture of membranes and preterm birth. J Obstet Gynaecol Res. 2012; 38:780–6.

114. Gulati S, Agrawal S, Raghunandan C, et al. Maternal serum interleukin-6 and its association with clinicopathological infectious morbidity in preterm premature rupture of membranes: a prospective cohort study. J Matern Fetal Neonatal Med. 2012; 25:1428–32.

115. Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Br J Obstet Gynaecol. 1995; 102:20–5.
116. Hamai Y, Fujii T, Yamashita T, et al. Evidence for an elevation in serum interleukin-2 and tumor necrosis factor-alpha levels before the clinical manifestations of preeclampsia. Am J Reprod Immunol. 1997; 38:89–93.
117. Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 1998; 40:102–11.

118. Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999; 180(2 Pt 1):499–506.

119. Sabatier F, Bretelle F, D’Ercole C, Boubli L, Sampol J, Dignat-George F. Neutrophil activation in preeclampsia and isolated intrauterine growth restriction. Am J Obstet Gynecol. 2000; 183:1558–63.

120. Gervasi MT, Chaiworapongsa T, Pacora P, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol. 2001; 185:792–7.

121. Teran E, Escudero C, Moya W, Flores M, Vallance P, Lopez-Jaramillo P. Elevated C-reactive protein and pro-inflammatory cytokines in Andean women with pre-eclampsia. Int J Gynaecol Obstet. 2001; 75:243–9.

122. Chaiworapongsa T, Romero R, Yoshimatsu J, et al. Soluble adhesion molecule profile in normal pregnancy and pre-eclampsia. J Matern Fetal Neonatal Med. 2002; 12:19–27.

123. Chaiworapongsa T, Gervasi MT, Refuerzo J, et al. Maternal lymphocyte subpopulations (CD45RA+ and CD45RO+) in preeclampsia. Am J Obstet Gynecol. 2002; 187:889–93.

124. Velzing-Aarts FV, Muskiet FA, van der Dijs FP, Duits AJ. High serum interleukin-8 levels in afro-caribbean women with pre-eclampsia: relations with tumor necrosis factor-alpha, duffy negative phenotype and von Willebrand factor. Am J Reprod Immunol. 2002; 48:319–22.
125. Serin IS, Ozcelik B, Basbug M, Kilic H, Okur D, Erez R. Predictive value of tumor necrosis factor alpha (TNF-alpha) in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2002; 100:143–5.
126. Belo L, Santos-Silva A, Caslake M, et al. Neutrophil activation and C-reactive protein concentration in preeclampsia. Hypertens Pregnancy. 2003; 22:129–41.

127. Levine RJ, Qian C, Leshane ES, et al. Two-stage elevation of cellfree fetal DNA in maternal sera before onset of preeclampsia. Am J Obstet Gynecol. 2004; 190:707–13.

128. Kocyigit Y, Atamer Y, Atamer A, Tuzcu A, Akkus Z. Changes in serum levels of leptin, cytokines and lipoprotein in pre-eclamptic and normotensive pregnant women. Gynecol Endocrinol. 2004; 19:267–73.
129. Freeman DJ, McManus F, Brown EA, et al. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension. 2004; 44:708–14.

130. Kim YM, Romero R, Oh SY, et al. Toll-like receptor 4: a potential link between “danger signals,” the innate immune system, and preeclampsia? Am J Obstet Gynecol. 2005; 193(3 Pt 2):921–7.

131. Braekke K, Holthe MR, Harsem NK, Fagerhol MK, Staff AC. Calprotectin, a marker of inflammation, is elevated in the maternal but not in the fetal circulation in preeclampsia. Am J Obstet Gynecol. 2005; 193:227–33.

132. Enquobahrie DA, Williams MA, Qiu C, Woelk GB, Mahomed K. Maternal plasma transforming growth factor-beta1 concentrations in preeclamptic and normotensive pregnant Zimbabwean women. J Matern Fetal Neonatal Med. 2005; 17:343–8.
133. Jonsson Y, Rubèr M, Matthiesen L, et al. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J Reprod Immunol. 2006; 70:83–91.

134. Kusanovic JP, Romero R, Hassan SS, et al. Maternal serum soluble CD30 is increased in normal pregnancy, but decreased in preeclampsia and small for gestational age pregnancies. J Matern Fetal Neonatal Med. 2007; 20:867–78.

135. Sharma A, Satyam A, Sharma JB. Leptin, IL-10 and inflammatory markers (TNF-alpha, IL-6 and IL-8) in pre-eclamptic, normotensive pregnant and healthy non-pregnant women. Am J Reprod Immunol. 2007; 58:21–30.
136. Laskowska M, Laskowska K, Leszczyn´ska-Gorzelak B, Oleszczuk J. Comparative analysis of the maternal and umbilical interleukin-8 levels in normal pregnancies and in pregnancies complicated by preeclampsia with intrauterine normal growth and intrauterine growth retardation. J Matern Fetal Neonatal Med. 2007; 20:527–32.

137. Sibai B, Romero R, Klebanoff MA, et al. Maternal plasma concentrations of the soluble tumor necrosis factor receptor 2 are increased prior to the diagnosis of preeclampsia. Am J Obstet Gynecol. 2009; 200:630:e1–8.

138. Ogge G, Romero R, Chaiworapongsa T, et al. Leukocytes of pregnant women with small-for-gestational age neonates have a different phenotypic and metabolic activity from those of women with preeclampsia. J Matern Fetal Neonatal Med. 2010; 23:476–87.
139. Soto E, Romero R, Richani K, et al. Preeclampsia and pregnancies with small-for-gestational age neonates have different profiles of complement split products. J Matern Fetal Neonatal Med. 2010; 23:646–57.

140. Szarka A, Rigó J Jr, Lázár L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010; 11:59.

141. Xie C, Yao MZ, Liu JB, Xiong LK. A meta-analysis of tumor necrosis factor-alpha, interleukin-6, and interleukin-10 in preeclampsia. Cytokine. 2011; 56:550–9.

142. Twina G, Sheiner E, Shahaf G, et al. Lower circulation levels and activity of alpha-1 antitrypsin in pregnant women with severe preeclampsia. J Matern Fetal Neonatal Med. 2012; 25:2667–70.
143. Stampalija T, Chaiworapongsa T, Romero R, et al. Maternal plasma concentrations of sST2 and angiogenic/anti-angiogenic factors in preeclampsia. J Matern Fetal Neonatal Med. 2013; 26:1359–70.

144. Sahin S, Ozakpinar OB, Eroglu M, et al. The impact of platelet functions and inflammatory status on the severity of preeclampsia. J Matern Fetal Neonatal Med. 2015; 28:643–8.

145. Labarrere C, Manni J, Salas P, Althabe O. Intrauterine growth retardation of unknown etiology. I. Serum complement and circulating immune complexes in mothers and infants. Am J Reprod Immunol Microbiol. 1985; 8:87–93.

146. Labarrere CA, Althabe OH. Intrauterine growth retardation of unknown etiology: II. Serum complement and circulating immune complexes in maternal sera and their relationship with parity and chronic villitis. Am J Reprod Immunol Microbiol. 1986; 12:4–6.

147. Johnston TA, Greer IA, Dawes J, Calder AA. Neutrophil activation in small for gestational age pregnancies. Br J Obstet Gynaecol. 1991; 98:105–6.

148. Johnson MR, Anim-Nyame N, Johnson P, Sooranna SR, Steer PJ. Does endothelial cell activation occur with intrauterine growth restriction? BJOG. 2002; 109:836–9.

149. Coata G, Pennacchi L, Bini V, Liotta L, Di Renzo GC. Soluble adhesion molecules: marker of pre-eclampsia and intrauterine growth restriction. J Matern Fetal Neonatal Med. 2002; 12:28–34.

150. Tjoa ML, van Vugt JM, Go AT, Blankenstein MA, Oudejans CB, van Wijk IJ. Elevated C-reactive protein levels during first trimester of pregnancy are indicative of preeclampsia and intrauterine growth restriction. J Reprod Immunol. 2003; 59:29–37.

151. Cetin I, Cozzi V, Pasqualini F, et al. Elevated maternal levels of the long pentraxin 3 (PTX3) in preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol. 2006; 194:1347–53.

152. Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. 2006; 203:2165–75.

153. Laskowska M, Laskowska K, Leszczyn´ska-Gorzelak B, Oleszczuk J. Maternal and umbilical sTNF-R1 in preeclamptic pregnancies with intrauterine normal and growth retarded fetus. Hypertens Pregnancy. 2007; 26:13–21.

154. Erez O, Romero R, Hoppensteadt D, et al. Tissue factor and its natural inhibitor in pre-eclampsia and SGA. J Matern Fetal Neonatal Med. 2008; 21:855–69.

156. Gilstrap LC 3rd, Lucas MJ. Urinary tract infections in women. Curr Opin Obstet Gynecol. 1990; 2:643–8.
157. Soto E, Richani K, Romero R, et al. Increased concentration of the complement split product C5a in acute pyelonephritis during pregnancy. J Matern Fetal Neonatal Med. 2005; 17:247–52.

158. Rajamäki K, Lappalainen J, Oörni K, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010; 5:e11765.

159. Small DM, Shipley GG. Physical-chemical basis of lipid deposition in atherosclerosis. Science. 1974; 185:222–9.

160. Sattar N, Bendomir A, Berry C, Shepherd J, Greer IA, Packard CJ. Lipoprotein subfraction concentrations in preeclampsia: pathogenic parallels to atherosclerosis. Obstet Gynecol. 1997; 89:403–8.

161. Lorentzen B, Endresen MJ, Hovig T, Haug E, Henriksen T. Sera from preeclamptic women increase the content of triglycerides and reduce the release of prostacyclin in cultured endothelial cells. Thromb Res. 1991; 63:363–72.

162. Hubel CA, McLaughlin MK, Evans RW, Hauth BA, Sims CJ, Roberts JM. Fasting serum triglycerides, free fatty acids, and malondialdehyde are increased in preeclampsia, are positively correlated, and decrease within 48 hours post partum. Am J Obstet Gynecol. 1996; 174:975–82.

163. Belo L, Santos-Silva A, Quintanilha A, Rebelo I. Similarities between pre-eclampsia and atherosclerosis: a protective effect of physical exercise? Curr Med Chem. 2008; 15:2223–9.

164. Lau SY, Guild SJ, Barrett CJ, et al. Tumor necrosis factor-alpha, interleukin-6, and interleukin-10 levels are altered in preeclampsia: a systematic review and meta-analysis. Am J Reprod Immunol. 2013; 70:412–27.

165. Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014; 10:466–80.

167. Potter JM, Nestel PJ. The hyperlipidemia of pregnancy in normal and complicated pregnancies. Am J Obstet Gynecol. 1979; 133:165–70.

168. Baker AM, Klein RL, Moss KL, Haeri S, Boggess K. Maternal serum dyslipidemia occurs early in pregnancy in women with mild but not severe preeclampsia. Am J Obstet Gynecol. 2009; 201: 293:e1–4.

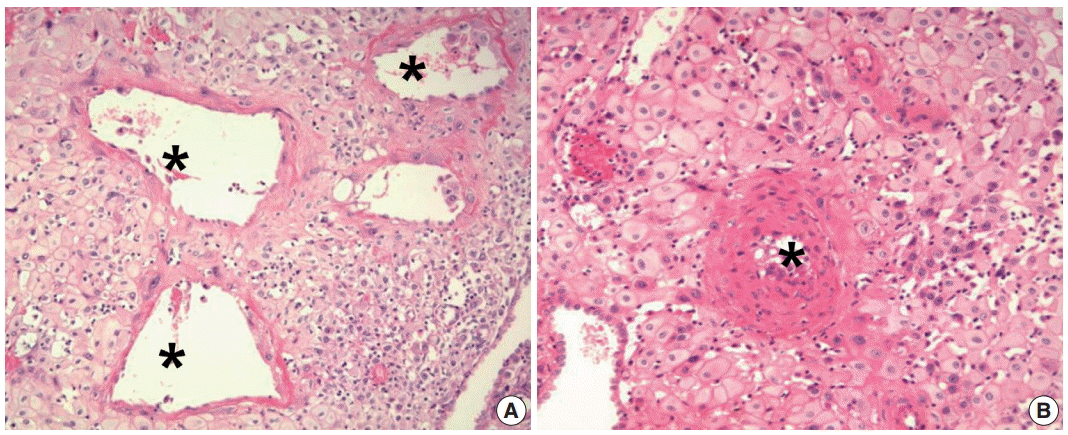
Fig. 1.
Spiral artery changes during pregnancy. (A) Normal physiologic transformation of spiral arteries in a normal pregnancy. The lumen of the spiral artery (asterisks) is dilated. Trophoblastic cells are infiltrating the wall of the spiral artery. (B) Failure of physiologic transformation of spiral arteries in a patient with preeclampsia. The lumen of the arteries (asterisk) is not dilated. The medial layers of the spiral arteries are intact. Although many interstitial trophoblasts surround the spiral artery, trophoblasts have not invaded the vessel wall.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download