CLINICAL FEATURES
NEUROIMAGING
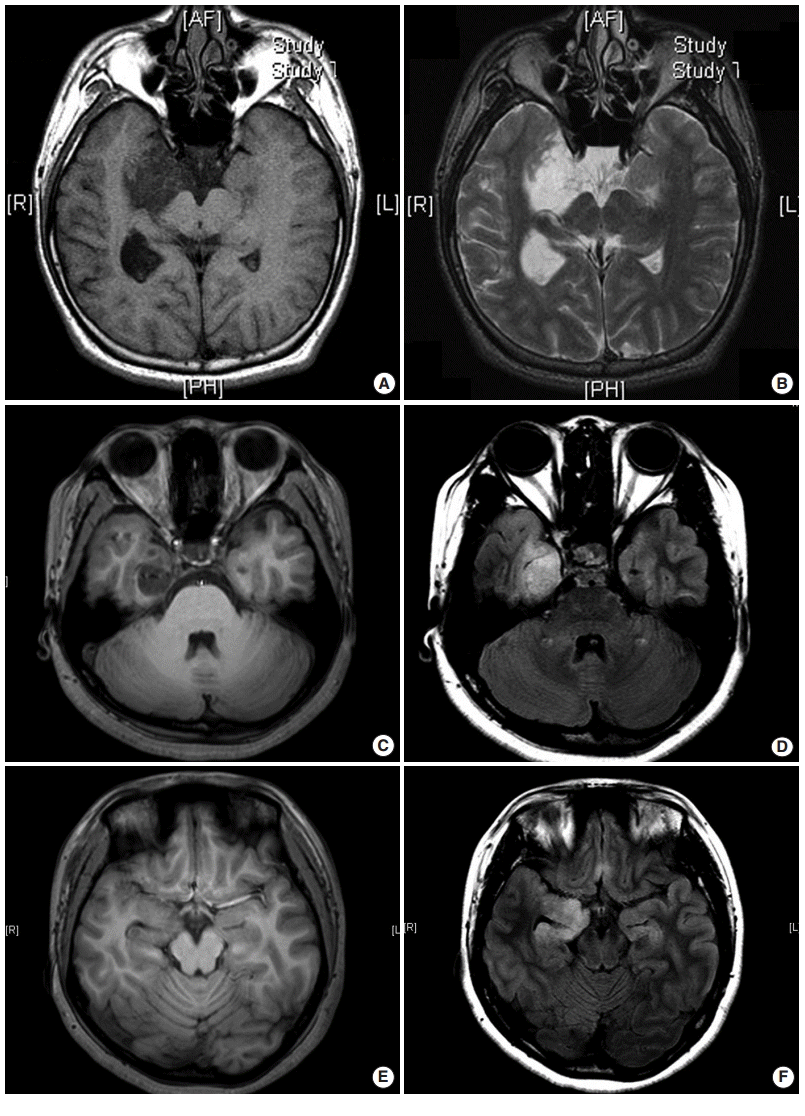 | Fig. 1.Three different types of magnetic resonance imaging in dysembryoplastic neuroepithelial tumors. (A, B) Type 1 shows a well-delineated, polycystic-like tumor with strongly hypointense on T1- and hyperintense on T2-weighted images. (C, D) Type 2 shows a nodular-like, heterogeneous lesion. (E, F) Type 3 shows a poorly delineated, dysplastic-like, iso/hyposignal T1 with gray-white matter blurring. Type 1 is mainly found in simple or complex forms, and type 2 and 3 are observed in nonspecific forms. (A, C, E) T1-weighted images. (B, D, F) T2 FLARE images. |
HISTOPATHOLOGY
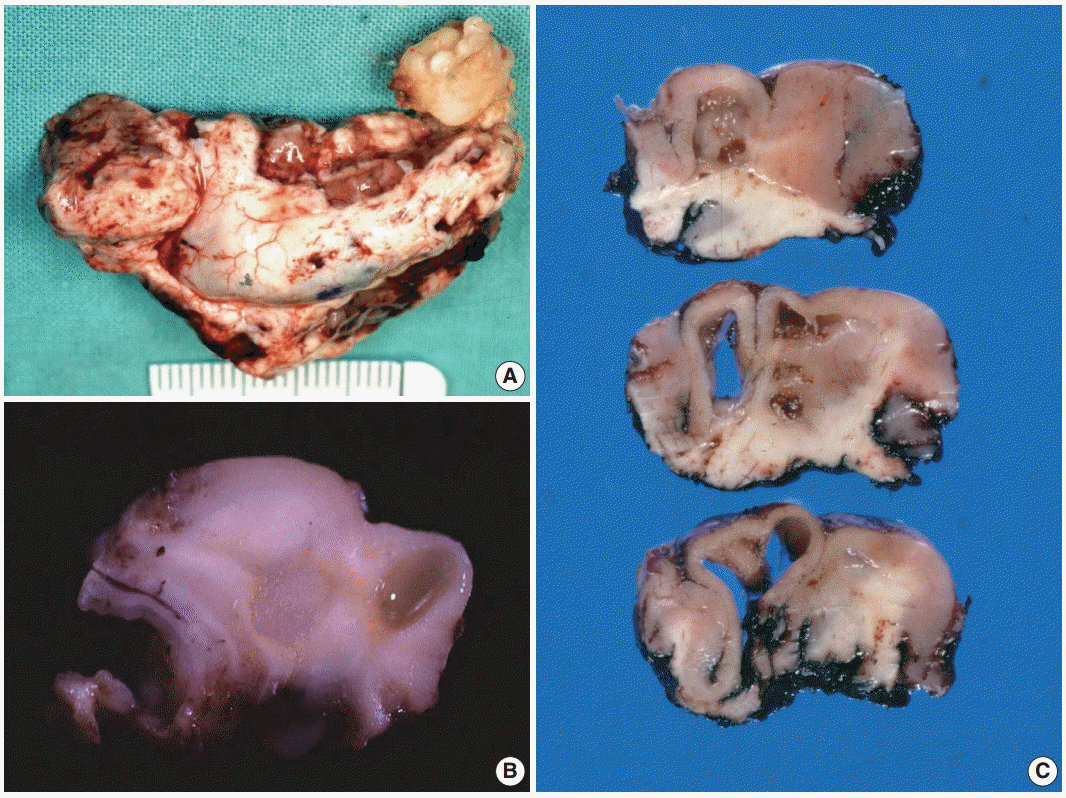 | Fig. 2. Gross findings of dysembryoplastic neuroepithelial tumors (DNTs). (A) A hippocampectomy specimen shows a well circumscribed, gray white mass with two small satellite nodules. (B) On the cut section, a complex type of DNT shows multiple gray-white or gelatinous nodules affecting the cortex and white matter. (C) Nonspecific DNT shows a poorly demarcated, cortical thickening with underlying area of white matter rarefaction and cyst formation. |
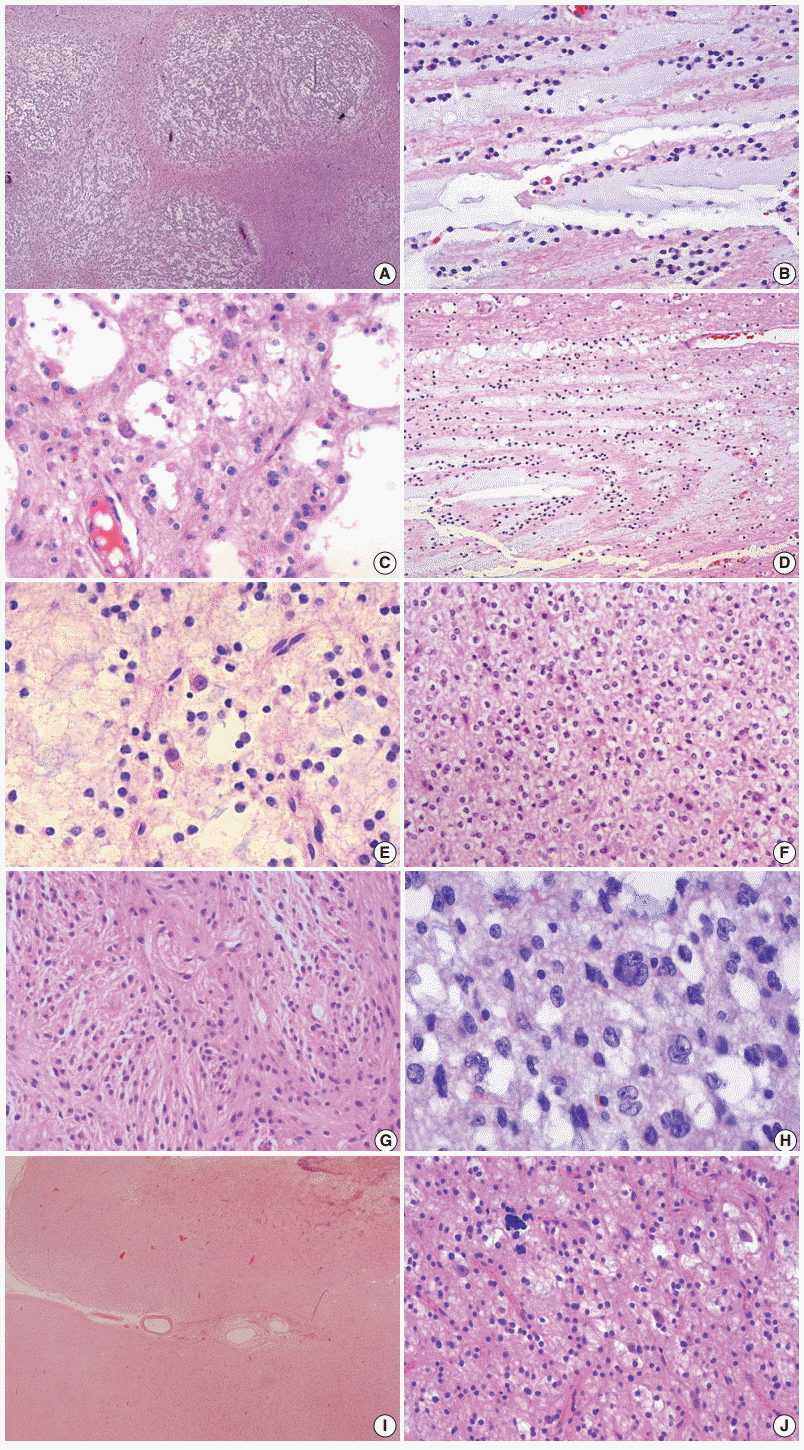 | Fig. 3.Histopathological findings of dysembryoplastic neuroepithelial tumors (DNTs). (A) Multi-nodular appearance typical of complex DNTs. (B) Column arrangement of oligodendroglioma-like tumor cells (OLCs) in the specific glioneuronal element. (C) The characteristic appearance of DNTs with OLCs and mature neurons. (D) Targetoid structure of the specific glioneuronal element. (E) Floating neurons in the mucinous matrix. (F) The histology of glial nodules resembling oligodendroglioma. (G) This glial nodule with predominantly astrocytic differentiation. (H)Nuclear pleomorphism in glial component of DNTs. Histopathological findings of dysembryoplastic neuroepithelial tumors (DNTs). (I) A poorly demarcated cortical lesion of nonspecific DNT. (J) The similar histology of nonspecific DNTs to that observed within the glial nodules of complex DNTs. |
IMMUNOHISOCHEMISTRY
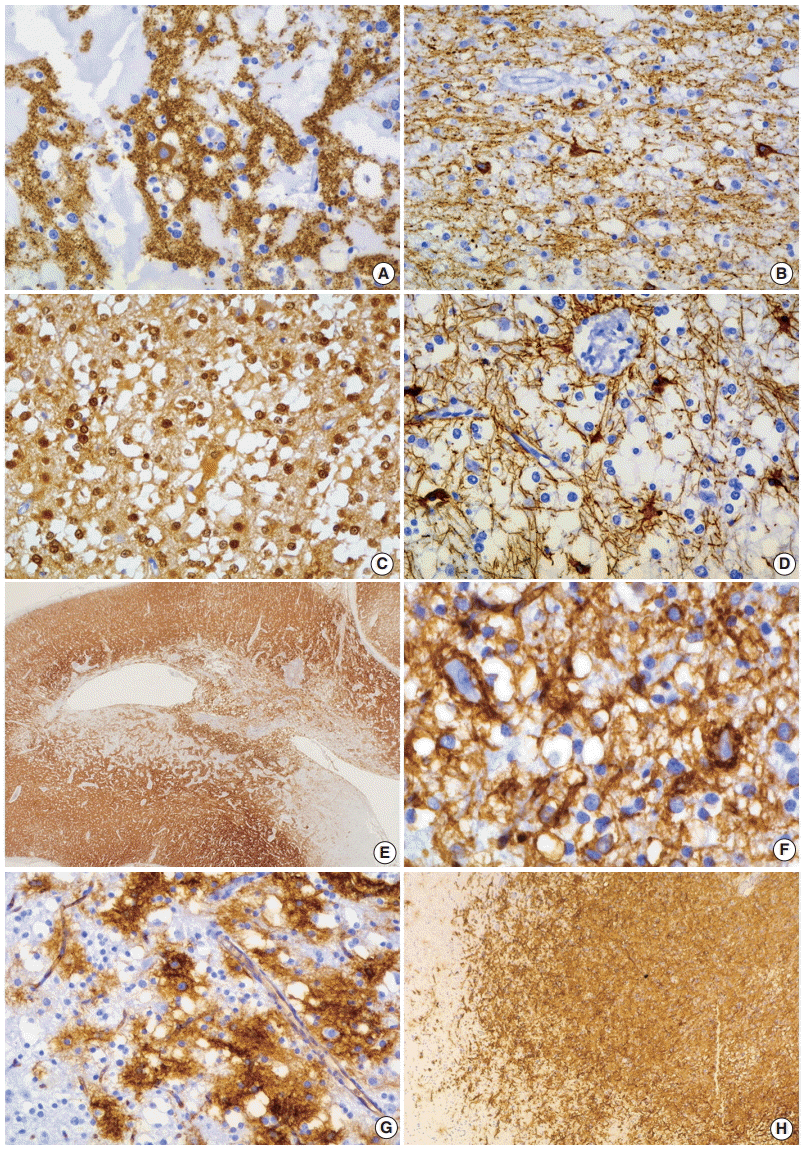 | Fig. 4. Immunohistochemical findings of dysembryoplastic neuroepithelial tumors (DNTs). (A, B) Floating neurons are positive for synaptophysin (A) and phosphorylated neurofilament (B). (C, D) The oligodendroglioma-like cells (OLCs) are diffusely positive for S-100 (C) but negative for glial fibrillary acidic protein (D). (E) Nonspecific DNTs show slightly decreased synaptophysin granular staining compared with that seen in the adjacent normal cortex. (F) In the specific glioneuronal element, CD34 is expressed along the perikarya and in membrane of the floating neurons, pericellular stroma, and cytoplasm of OLCs. (G, H) The specific glioneuronal element shows cluster staining pattern of CD34 (G), while diffuse CD34 immunoreactivity in glial nodules of complex DNTs (H). |
ULTRASTRUCTURAL FINDINGS
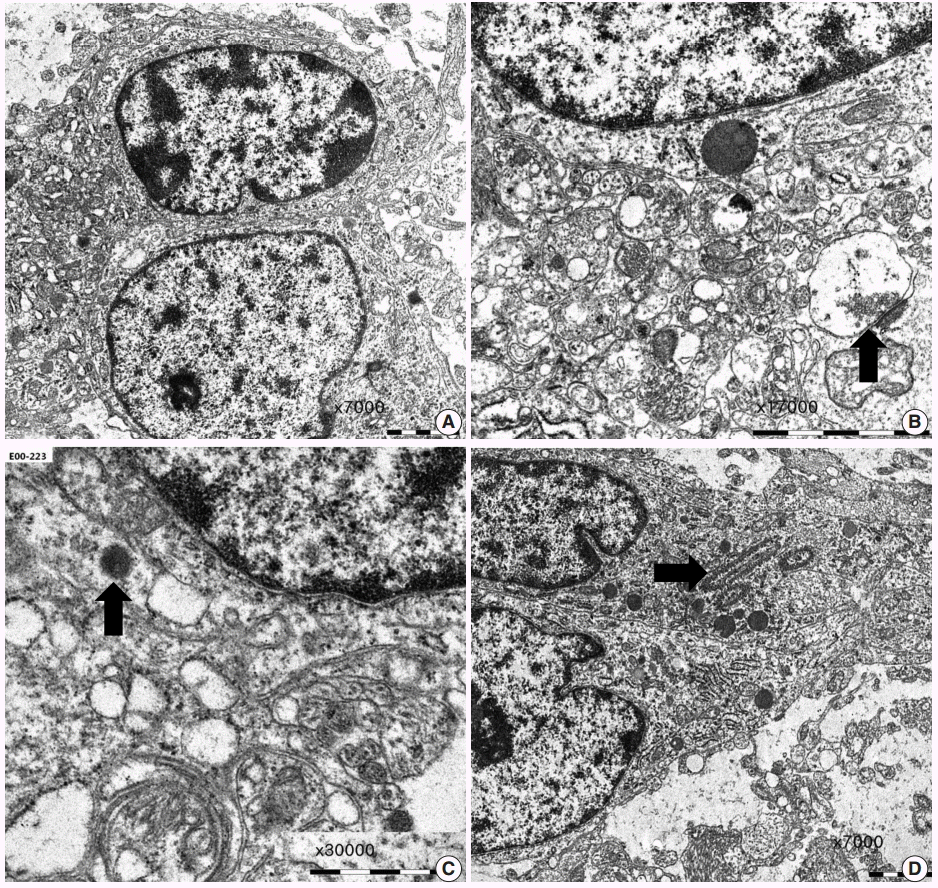 | Fig. 6. Ultrastructural findings of dysembryoplastic neuroepithelial tumors. (A) Oligodendroglioma-like cells (OLCs) show oval nuclei with small indentation, marginal aggregates of heterochromatin, and their cytoplasm with scanty organelles (×7,000). (B, C) Neuropil-like network of cellular process with a synaptic contact (arrow) (B, ×17,000) and scant dense core granules (arrow) (C, ×30,000) indicates neuronal differentiation of OLCs. (D) A few OLCs contain ribosome-lamellae complex inclusions (arrow) (×7,000). |
SQUASH CYTOLOGICAL FEATURES
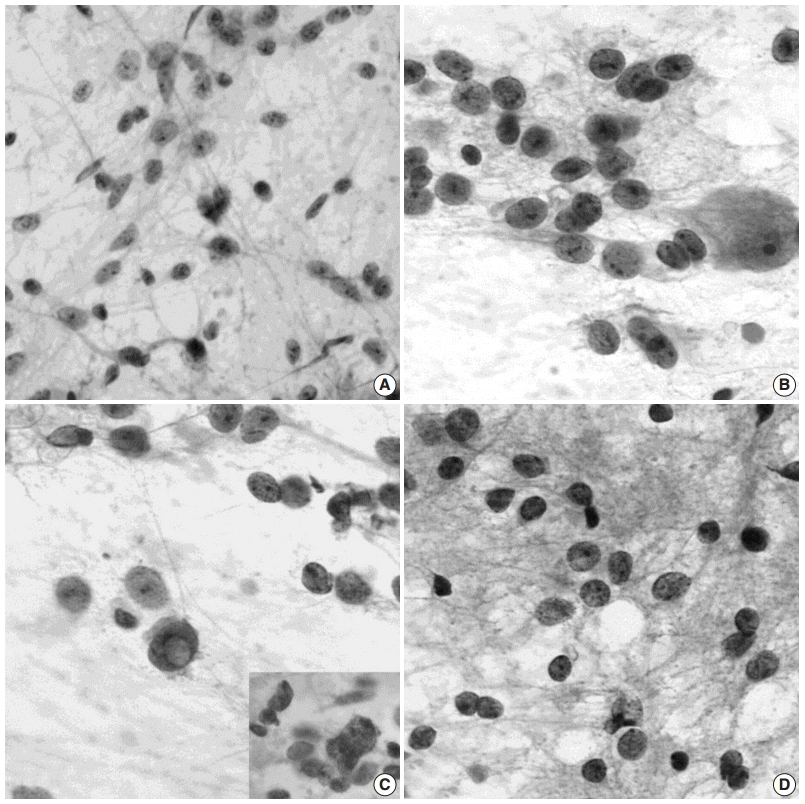 | Fig. 7.Squash cytological findings of dysembryoplastic neuroepithelial tumors. (A) Squash preparation shows round, oval to elongated naked nuclei in the mucinous background. (B) The nuclei of oligodendroglioma-like cells (OLCs) are irregular with small indentations or deep grooves, and fine, granular chromatin and 1–4 small nucleoli. There is a large, normal-looking neuron in the mucinous background. (C) OLCs shows multinuclear giant cell formation (inset) and an intranuclear pseudoinclusion. (D) Squash preparation of oligodendroglioma shows smaller, dark nuclei without nucleoli and larger nuclei with granular chromatin and micronucleoli. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download