Abstract
Background:
Chronic placental inflammation, such as villitis of unknown etiology (VUE) and chronic chorioamnionitis (CCA), is considered a placental manifestation of maternal anti-fetal rejection. The aim of this study is to investigate its frequency in twin pregnancies compared to singleton pregnancies.
Methods:
Three hundred twin placentas and 1,270 singleton placentas were consecutively collected at a tertiary medical center in Seoul, Republic of Korea from 2009 to 2012. Hematoxylin and eosin sections of tissue samples (full-thickness placental disc and chorioamniotic membranes) were reviewed.
Results:
Non-basal VUE was more frequent in twin placentas than in singleton placentas (6.0% vs 3.2%, p < .05). In preterm birth, CCA was found less frequently in twin placentas than in singleton placentas (9.6% vs 14.8%, p < .05), reaching its peak at an earlier gestational age in twin placentas (29–32 weeks) than in singleton placentas (33–36 weeks). CCA was more frequent in twin pregnancies with babies of a different sex than with those with the same sex (13.8% vs 6.9%, p=.052). Separate dichorionic diamniotic twin placentas were affected by chronic deciduitis more frequently than singleton placentas (16.9% vs 9.7%, p<.05).
Conclusions:
The higher frequency of non-basal VUE in twin placentas and of CCA in twin placentas with different fetal sex supports the hypothesis that the underlying pathophysiological mechanism is maternal anti-fetal rejection related to increased fetal antigens in twin pregnancies. The peak of CCA at an earlier gestational age in twin placentas than in singleton placentas suggests that CCA is influenced by placental maturation.
Chronic inflammation of the placenta can be present in the chorionic villous tree, extraplacental chorioamniotic membranes, and the basal plate of the placenta, which are called chronic villitis, chronic chorioamnionitis (CCA), and chronic deciduitis (CD), respectively. Infection may cause some of the chronic placental inflammation; however, infectious microorganisms are not identified in a majority of cases. Chronic villitis in such cases is called villitis of unknown etiology (VUE). It is defined by the infiltration of maternal lymphocytes into the fetal chorionic villi, and we have shown that fetal placental macrophages also participate in VUE [1]. Its prevalence is reported to range from 2% to 33.8% [2], and it is more frequent in placentas at term than at preterm. VUE at term is usually subclinical, while high-grade or diffuse VUE is associated with fetal growth restriction, intrauterine fetal death, or fetal central nervous system injury [3,4]. CCA is characterized by maternal lymphocytic infiltration into the chorioamniotic membranes and chorionic plate [5,6]. A series of recent studies have demonstrated that CCA is another major pathology of preterm birth, especially of late preterm births [7,8]. CCA is also frequently found in intrauterine fetal death cases [9]. CD is defined by the accumulation of lymphocytes and plasma cells in the decidua of the basal plate [10] and often accompanies basal VUE that involves anchoring villi. It is associated with fetal growth restriction and preterm labor [11-14].
The fetus and placenta are semi-allografts to the mother. Immune tolerance is required for successful pregnancy [15,16]. Accumulating evidence from recent studies suggests that maternal anti-fetal rejection may explain the pathogenesis of VUE and CCA [1,7,17-19]. An immune origin of CD has also been suggested, as this condition has been reported more frequently in pregnancies resulting from donor egg in vitro fertilization pregnancies [20,21].
The rate of multiple pregnancies has increased over the past three decades to reach 33.7 per 1,000 total births in the United States in 2013, which results from the expanded use of fertility enhancing therapies and an older maternal age at childbearing [22]. Infants born in multiple gestation pregnancies are at a higher risk of adverse birth outcomes than singletons mainly because of the increased frequency of preterm birth in addition to twin-specific complications, such as twin-to-twin transfusion syndrome and discordant growth restriction [22]. A thorough investigation of chronic placental inflammation in twin placentas has not been performed, though some reports have focused on the relationship between VUE and intrauterine growth restriction (IUGR) in twin placentas [23-25].
In this study, we compared the frequencies of chronic placental inflammation, VUE, CCA, and CD between twin and singleton placentas collected consecutively at a tertiary medical center in order to investigate whether chronic inflammation is more frequent in twin placentas because of the increased burden of fetal semi-allograft antigens.
Three hundred twin placentas (77 separate dichorionic diamniotic [DiDiS], 147 fused dichorionic diamniotic [DiDiF], 66 monochorionic diamniotic [MoDi], 8 monochorionic monoamniotic [MoMo], and 2 unknown type) and 1,270 singleton placentas were consecutively collected at Samsung Medical Center, Seoul, Republic of Korea, from 2009 to 2012. The study was approved by the institutional review board of the hospital (2011-07-063).
Preterm birth was defined as delivery before 37 weeks of gestation and was categorized as (1) spontaneous preterm births resulting from preterm labor and intact membranes, and preterm premature rupture of membranes; and (2) indicated preterm births due to maternal and/or fetal conditions necessitating preterm births. IUGR was defined as birth weight below the 10th percentile, and discordant growth in twins was defined as more than 15% difference in birth weight.
Tissue samples of the full-thickness placental disc (n=2) and chorioamniotic membranes roll (n=1) for each placenta, and also of the dividing membranes if present, were obtained for microscopic examination and were formalin-fixed for 24 hours. The hematoxylin and eosin stained sections were reviewed for the presence of chronic inflammation. VUE, CCA, and CD were diagnosed according to the previously described criteria in the literature [7,10,17]. Briefly, VUE was defined as lymphohistiocytic infiltration affecting varying proportions of the villous tree of the placenta without any signs or symptoms of infection in either the mother or the infant [17,26]. It is sub-classified as basal type, which involves villi anchoring to the basal plate, and nonbasal (distal and proximal) type, which involves distal villi (terminal and mature intermediate villi) and/or stem villi. The diagnosis of CCA was made when there was lymphocytic infiltration into the chorionic trophoblast layer and/or chorioamniotic connective tissue [7]. CD was diagnosed when lymphocytic infiltration was diffuse and/or any plasma cells were detected in the decidua [10]. The cases were examined by a single pathologist (J.-S.K.) who was blind to clinical information.
The chi-square test and Fisher exact test were performed to compare proportions, and the Mann-Whitney U test was performed to determine the differences in the median values between the study groups. The SPSS ver. 21 (IBM Co., Armonk, NY, USA) was employed for all statistical analyses. A p-value of less than .05 was considered statistically significant.
The demographics and clinical characteristics of the study population are summarized in Table 1. There were significant differences in the gestational age at delivery, gravity, labor at delivery, preterm birth, and IUGR between singleton and twin pregnancies (p<.05). Maternal age, previous abortion, the ratio of spontaneous/indicated preterm birth, and hypertensive disorders were not significantly different.
The frequencies of the three types of chronic placental inflammation (VUE, CCA, and CD) were not statistically different between twin and singleton placentas. Twin placentas had a tendency of high frequency of VUE (11.3% [34/300] vs 7.9% [100/1,270], p=.054) and CD (13.0% [36/300] vs 9.7% [123/1,270], p=.09) compared with singleton placentas, whereas they showed a tendency of low frequency of CCA (9.3% [28/300] vs 13.3% [169/1,270], p=.062) (Fig. 1A, C, D). The frequency of the non-basal type of VUE was higher in twin placentas than in singleton placentas (6.0% [18/300] vs 3.2% [41/1,270], p<.05). There was no difference in the frequency of basal VUE between twin and singleton placentas (5.3% [16/300] vs 4.6% [59/1,270], p>.05) (Fig. 1B).
There were no significant differences in the frequencies of VUE (10.2% [23/225] vs 15.1% [11/73]), CCA (9.3% [21/225]vs 9.6% [7/73]), or CD (13.8% [31/225] vs 11.0% [8/73]) between dichorionic (DiDiS and DiDiF) and monochorionic (MoDi and MoMo) placentas (p>.05). CD was significantly more frequent in DiDiS twin placentas compared with singleton placentas (16.9% [13/77] vs. 9.7% [123/1,270], p<.05), but the frequencies of VUE and CCA were not significantly different among the twin types.
The frequency of VUE increased with increasing gestational age at birth in both singleton and twin placentas; however, there was no significant difference in the frequency of VUE between singleton and twin placentas when the cases were matched by gestational age (Fig. 2A). When the study population was divided into preterm and term births, term twin placentas were affected by non-basal VUE more frequently than term singleton placentas (12.9% [9/70] vs 3.6% [14/384], p<.01), but the difference was not detected in preterm placentas (Fig. 2B). The frequencies of basal VUE were not significantly different between singleton and twin placentas either at preterm or at term (Fig. 2C).
CCA was found less frequently in twin placentas than singleton placentas of preterm birth (9.6% [22/230] vs 14.8% [131/886], p<.05) (Fig. 3A). Among preterm births, the decrease of CCA in twin placentas compared with singleton placentas was significant at 33–36 weeks of gestation (late preterm birth) (10.0% [12/120] vs 17.9% [90/503], p<.05). CCA in singleton placentas was most commonly observed at late preterm stage (33–36 weeks of gestation) during gestation as previously reported [8]; however, CCA in twin placentas was most frequent at 29–32 weeks unexpectedly (14.0% [6/43]) (Fig. 3B).
VUE in singleton placentas was found more frequently in IUGR cases than in non-IUGR cases (12.9% [36/278] vs 6.5% [64/985], p<.001). Of these cases, non-basal VUE was more frequent in IUGR cases than in non-IUGR cases (7.6% [21/278] vs 2.0% [20/985]), p<.001). Non-basal VUE in twin placentas was more frequent in IUGR cases than in non-IUGR cases (9.8% [12/122] vs 3.4% [6/175], p<.05), though VUE showed no significant correlation with IUGR in twin placentas (13.1% [16/122] vs 10.3% [18/175]) (Fig. 5A, B). Neither CCA nor CD correlated with IUGR in this study (Fig. 5C, D).
VUE was more frequent in indicated preterm birth than in spontaneous birth of singleton placentas (10.2% [24/235] vs 6.3% [41/651], p<.05) (Fig. 6A). Non-basal VUE was more frequent in indicated preterm birth than in spontaneous birth of both singleton and twin placentas (singleton, 5.5% [13/235] vs 2.2% [14/651]; twin, 9.1% [5/55] vs 2.3% [4/175], p<.05) (Fig. 6B). When the frequencies of chronic placental inflammation were compared after preterm cases were divided into spontaneous and indicated preterm birth groups, none of the chronic inflammation conditions showed any statistically significant differences between singleton and twin placentas (Fig. 6A–D).
CCA showed a tendency toward higher frequency in twin pregnancies with babies of a different sex than with those of the same sex (13.8% [15/109] vs 6.9% [13/189], p=.052), whereas the frequencies of VUE and CD were not significantly different between twin pregnancies of a different sex and those of the same sex. The frequencies of chronic inflammation in twin placentas had no significant correlation with primigravida, previous abortion, intrauterine fetal death, or discordant growth (p>.05).
Chronic placental inflammation was detected in either one or both twin placentas (VUE, 7.3% [22/300] vs 4.0% [12/300]; CCA, 5.4% [16/300] vs 4.0% [12/300]; CD, 7.7% [23/300] vs 6.0% [18/300]). The presence of chronic inflammation in one or both twin placentas was not correlated with other clinical and placental characteristics, including baby sex, chorionicity, and discordant growth. There were 11 cases of IUGR in only one fetus of twin pregnancies with VUE, of which eight cases had VUE in only one placenta and the other three cases had VUE in both placentas, suggesting a tendency of correlation between IUGR and VUE that was not statistically significant.
In this study, the frequency of VUE, non-basal type, was higher in twin placentas than in singleton placentas. Non-basal VUE had a correlation with IUGR in twin placentas as in singleton placentas. Unexpectedly, CCA was found less frequently in twin placentas than in singleton placentas of preterm birth. The frequency peak of CCA in twin placentas was present at 29–32 weeks in contrast to that of singleton placentas at 33–36 weeks. CCA was more frequent in twin pregnancies of a different sex than in those of the same sex. The frequency of CD was higher in DiDiS twin placentas than in singleton placentas.
Chronic placental inflammation is present as chronic villitis, CCA, and CD, depending on the affected compartment of the placenta. Some of the chronic placental inflammation may be caused by infectious agents, such as viruses, bacteria, and parasites [2,27,28]. However, evidence of infectious etiology is not identified in most cases of chronic placental inflammation. Maternal anti-fetal rejection is suggested to play a role in the pathogenesis of these conditions. Destruction of chorionic villi (VUE) and chorionic trophoblast layer (CCA) of fetal origin by infiltration of maternal CD8+ T cells, along with activation of fetal macrophages, is the histopathological hallmark of these lesions [1,6,29]. The pathogenetic mechanism of a rejection process is also supported by the elevation of local and systemic T-cell chemokines and the presence of fetal HLA-specific antibodies in the maternal serum [7,17,18].
VUE of non-basal type was more frequent in twin placentas than singleton placentas, and the difference was more prominent at term when VUE was detected more often in singleton placentas. The increased fetal antigen burden in twin placentas could be related to its high frequency. In contrast, basal VUE was detected at a similar frequency between singleton and twin placentas. Basal VUE may be influenced partly by a different immunologic mechanism compared with non-basal VUE, which is supported by the presence of plasma cells in basal villitis as well as its association with CD. CD did not show a frequency difference between twin and singleton placentas in this study; however, the frequency of CD was increased in DiDiS twin placentas. DiDiS twin placentas are the type with the highest chance of dizygosity, which has more fetal antigens than the other types of twin placentas. This result suggests that it is also an anti-fetal rejection phenomenon, at least in part, which is relevant to the increase of CD in egg donor pregnancies [20,21]. The frequency of CCA was increased in twin placentas of a different sex, which also indicated the increased fetal antigen burden. Twin pregnancies with different fetal sex are definitely dizygotic, therefore these placentas could have higher allograft antigenicity compared with those with the same fetal sex. Further studies are required to elucidate why there was no significant difference in VUE and CD between twin placentas with different fetal sex and those with the same sex.
Unexpectedly, CCA was decreased in twin placentas compared with singleton placentas at preterm. As previously reported, CCA was most frequent at 33–36 weeks gestation in singleton placentas in this study [8], while the highest frequency of CCA in twin placentas was detected at 29–32 weeks of gestation, which is earlier than in singleton placentas. In general, maturation of twin placentas is accelerated compared to singleton placentas at the same gestational age. The period of vulnerability to CCA may be shifted earlier due to accelerated maturation of twin placentas. These results suggest that CCA is also a manifestation of host anti-graft rejection, but a maturationrelated factor may play a role in CCA.
The association of VUE with IUGR is well known by previous studies [28,30-32]. In this study, it was confirmed in singleton placentas, and the non-basal type of VUE was associated with IUGR in twin placentas. This result also explains the high frequency of VUE in indicated preterm birth, which included most of the IUGR cases. Several previous reports showed that villitis was more severe in the placenta of the twin that weighed less, though the correlation was not confirmed statistically [23-25]. In this study, the degree of VUE was not included for analysis. The absence of a significant association of IUGR with the total VUE in twin placentas might be influenced by various twin-specific factors resulting in IUGR, such as limitation of the uterine space, insufficient uteroplacental function, twin-to-twin transfusion syndrome, and so on [33].
This study has some limitations. First, the enrollment of the study population was not prospective or fully consecutive, because some placentas from normal term pregnancies were not included. The institution where the placentas were collected was a tertiary medical center that takes care of more complicated cases; therefore, the cases likely do not represent the pregnancies of the general population. Second, the twin placentas were subdivided according to chorionicity but not zygosity, which would more reliably represent an increased antigen burden. These limitations might make the results less significant. However, it was clearly demonstrated that non-basal VUE was increased in twin placentas, and that CCA was decreased in preterm twin placentas. It is suggested that VUE and CCA are the region-specific manifestations of maternal anti-fetal rejection in the placenta, but their pathophysiology are partly different. Further studies are required to investigate the difference.
ACKNOWLEDGMENTS
The authors are very grateful to Professor Je G. Chi, who passed away last winter (November 26, 2014). He was a great mentor and friend to all of us. This work is dedicated to his memory.
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2012R1A1A3012668), and by the Korean Health Technology R&D Project, Ministry of Health & Welfare (HI1200024 (A120035)), Republic of Korea.
REFERENCES
1. Kim JS, Romero R, Kim MR, et al. Involvement of Hofbauer cells and maternal T cells in villitis of unknown aetiology. Histopathology. 2008; 52:457–64.

3. Derricott H, Jones RL, Heazell AE. Investigating the association of villitis of unknown etiology with stillbirth and fetal growth restriction: a systematic review. Placenta. 2013; 34:856–62.
4. Redline RW, Ariel I, Baergen RN, et al. Fetal vascular obstructive lesions: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2004; 7:443–52.

5. Gersell DJ, Phillips NJ, Beckerman K. Chronic chorioamnionitis: a clinicopathologic study of 17 cases. Int J Gynecol Pathol. 1991; 10:217–29.
6. Jacques SM, Qureshi F. Chronic chorioamnionitis: a clinicopathologic and immunohistochemical study. Hum Pathol. 1998; 29:1457–61.

7. Kim CJ, Romero R, Kusanovic JP, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol. 2010; 23:1000–11.

8. Lee J, Kim JS, Park JW, et al. Chronic chorioamnionitis is the most common placental lesion in late preterm birth. Placenta. 2013; 34:681–9.

9. Lee J, Romero R, Dong Z, et al. Unexplained fetal death has a biological signature of maternal anti-fetal rejection: chronic chorioamnionitis and alloimmune anti-human leucocyte antigen antibodies. Histopathology. 2011; 59:928–38.

10. Khong TY, Bendon RW, Qureshi F, et al. Chronic deciduitis in the placental basal plate: definition and interobserver reliability. Hum Pathol. 2000; 31:292–5.
11. Bendon RW, Miller M. Routine pathological examination of placentae from abnormal pregnancies. Placenta. 1990; 11:369–70.
12. Edmondson N, Bocking A, Machin G, Rizek R, Watson C, Keating S. The prevalence of chronic deciduitis in cases of preterm labor without clinical chorioamnionitis. Pediatr Dev Pathol. 2009; 12:16–21.

13. Yoo C, Jang DG, Jo YS, Yoo JL, Lee G. Pathologic differences between placentas from intrauterine growth restriction pregnancies with and without absent or reversed end diastolic velocity of umbilical arteries. Korean J Pathol. 2011; 45:36–44.

14. Park SY, Kim MY, Kim YJ, et al. Placental pathology in intrauterine growth retardation. Korean J Pathol. 2002; 36:30–7.
15. Trowsdale J, Betz AG. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. 2006; 7:241–6.

16. Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol. 2013; 13:23–33.

17. Kim MJ, Romero R, Kim CJ, et al. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J Immunol. 2009; 182:3919–27.

18. Lee J, Romero R, Xu Y, et al. Maternal HLA panel-reactive antibodies in early gestation positively correlate with chronic chorioamnionitis: evidence in support of the chronic nature of maternal anti-fetal rejection. Am J Reprod Immunol. 2011; 66:510–26.

19. Lee J, Romero R, Xu Y, et al. A signature of maternal anti-fetal rejection in spontaneous preterm birth: chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PLoS One. 2011; 6:e16806.

20. Perni SC, Predanic M, Cho JE, Baergen RN. Placental pathology and pregnancy outcomes in donor and non-donor oocyte in vitro fertilization pregnancies. J Perinat Med. 2005; 33:27–32.

21. Gundogan F, Bianchi DW, Scherjon SA, Roberts DJ. Placental pathology in egg donor pregnancies. Fertil Steril. 2010; 93:397–404.

22. Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015; 64:1–65.
23. Eberle AM, Levesque D, Vintzileos AM, Egan JF, Tsapanos V, Salafia CM. Placental pathology in discordant twins. Am J Obstet Gynecol. 1993; 169:931–5.

24. Jacques SM, Qureshi F. Chronic villitis of unknown etiology in twin gestations. Pediatr Pathol. 1994; 14:575–84.

25. Yusuf K, Kliman HJ. The fetus, not the mother, elicits maternal immunologic rejection: lessons from discordant dizygotic twin placentas. J Perinat Med. 2008; 36:291–6.

26. Redline RW. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Hum Pathol. 2007; 38:1439–46.

27. Altshuler G, Russell P. The human placental villitides: a review of chronic intrauterine infection. Curr Top Pathol. 1975; 60:64–112.

28. Becroft DM, Thompson JM, Mitchell EA. Placental villitis of unknown origin: epidemiologic associations. Am J Obstet Gynecol. 2005; 192:264–71.

29. Redline RW, Patterson P. Villitis of unknown etiology is associated with major infiltration of fetal tissue by maternal inflammatory cells. Am J Pathol. 1993; 143:473–9.
30. Knox WF, Fox H. Villitis of unknown aetiology: its incidence and significance in placentae from a British population. Placenta. 1984; 5:395–402.

31. Redline RW, Patterson P. Patterns of placental injury. Correlations with gestational age, placental weight, and clinical diagnoses. Arch Pathol Lab Med. 1994; 118:698–701.
Fig. 1.
The frequency of chronic placental inflammation in twin placentas. (A) The frequency of villitis of unknown etiology (VUE) is not statistically different between twin and singleton placentas. (B) Non-basal type of VUE is more frequent in twin placentas than in singleton placentas (*p<.05), but basal type of VUE is not. (C, D) The frequencies of chronic chorioamnionitis (CCA) (C) and chronic deciduitis (CD) (D) are not statistically different between twin and singleton placentas.
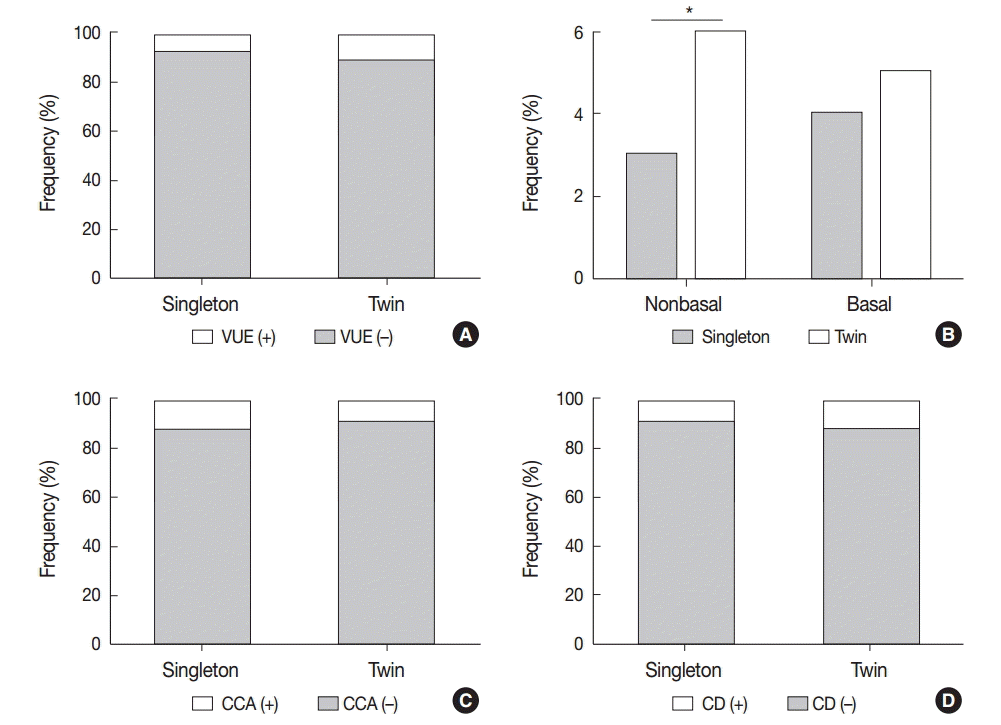
Fig. 2.
The frequency of villitis of unknown etiology (VUE) in twin placentas according to the gestational age. (A) VUE in singleton and twin placentas is found more frequently as gestational age at birth increases. (B) Non-basal VUE is more frequent in twin placentas than in singleton placentas at term (**p<.01). (C) There is no significant difference in the frequency of basal VUE between singleton and twin placentas. GAD, gestational age at delivery
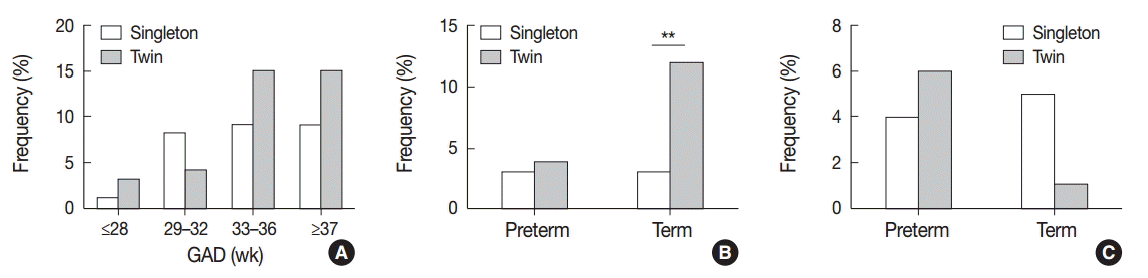
Fig. 3.
The frequency of chronic chorioamnionitis (CCA) in twin placentas according to the gestational age. (A) CCA is less frequent in twin placentas than singleton placentas at preterm birth (*p<.05). (B) CCA in twin placentas is decreased significantly compared with singleton placentas at 33–36 weeks of gestation (*p<.05). Twin placentas are affected by CCA most frequently at 29–32 weeks, in contrast to singleton placentas at 33–36 weeks. GAD, gestational age at delivery
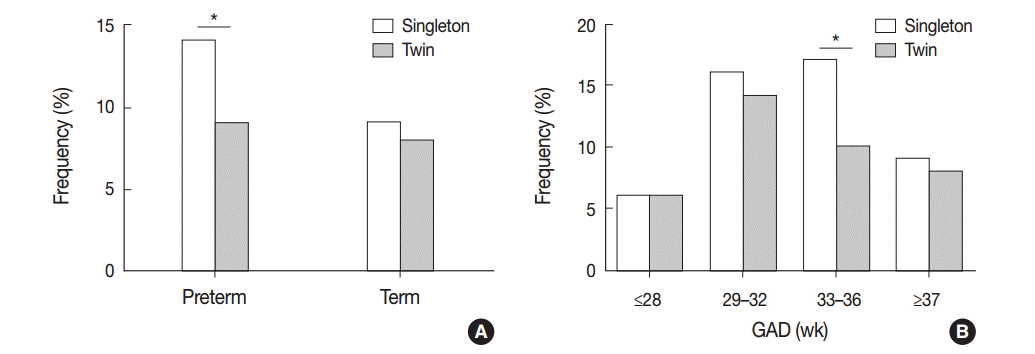
Fig. 4.
The frequency of chronic deciduitis (CD) in twin placentas at preterm and term birth. (A) CD is detected at similar frequencies between preterm and term placentas. (B) Separate dichorionic diamniotic (DiDiS) twin placentas are affected by CD more frequently than singleton placentas, especially at term (**p<.01).
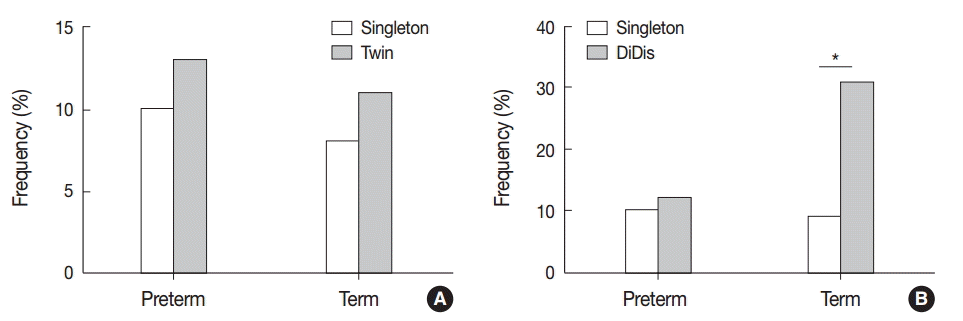
Fig. 5.
Association of villitis of unknown etiology (VUE) with intrauterine growth restriction (IUGR). (A) VUE is found more frequently in singleton placentas of IUGR cases than in those of non-IUGR cases (***p<.001). (B) Non-basal VUE in singleton and twin placentas is more frequent in IUGR cases than in non-IUGR cases (singleton, ***p<.001; twin, *p<.05). (C, D) The frequencies of chronic chorioamnionitis (C) and chronic deciduitis (D) are not associated with IUGR.
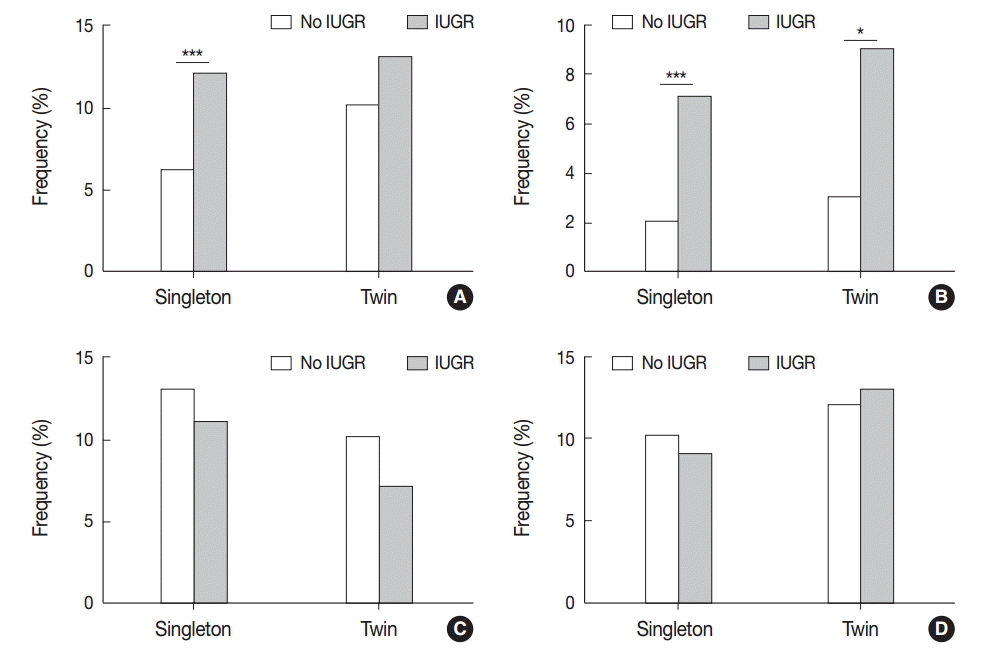
Fig. 6.
The frequency of chronic placental inflammation in spontaneous preterm birth and indicated preterm birth. (A) Villitis of unknown etiology (VUE) is more frequent in indicated preterm birth than in spontaneous birth of singleton placentas (*p<.05). (B) Non-basal VUE is detected more frequently in indicated preterm birth than in spontaneous birth of singleton placentas as well as twin placentas (*p<.05). (C, D) The frequencies of chronic chorioamnionitis (C) and chronic deciduitis (D) are not different between spontaneous and indicated preterm birth.
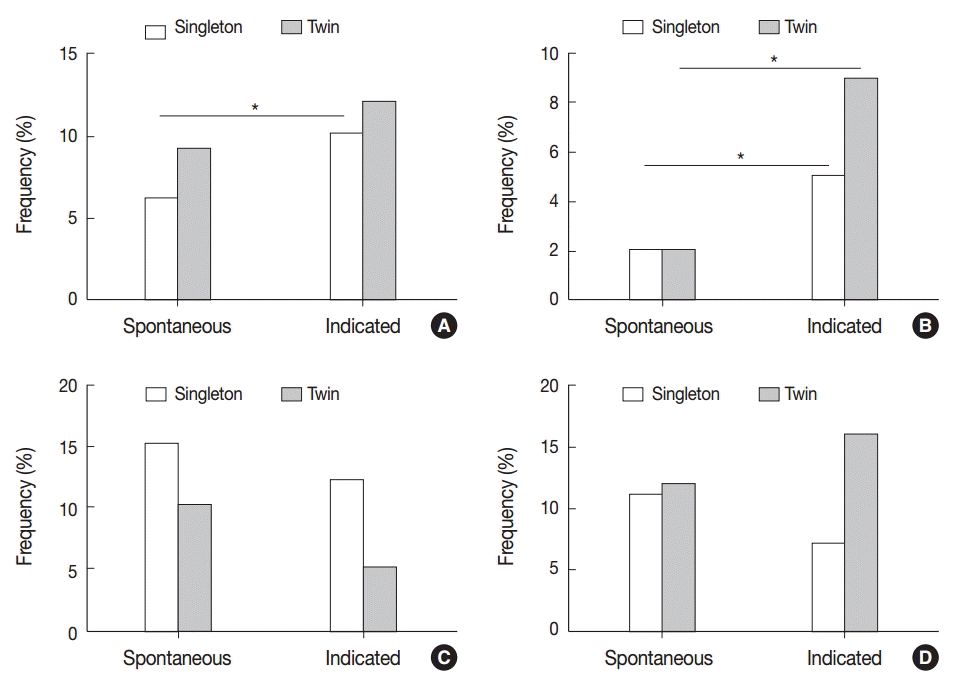
Table 1.
Clinical characteristics of the study groups




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download