Abstract
Background:
Cytologic diagnosis of pulmonary adenoid cystic carcinoma (AdCC) is frequently challenging and differential diagnosis with small cell carcinoma is often difficult.
Methods:
Eleven cytologically diagnosed cases of pulmonary AdCC were collected and reviewed according to fifteen cytomorphologic characteristics: small cell size, cellular uniformity, coarse chromatin, hyperchromasia, distinct nucleolus, frequent nuclear molding, granular cytoplasm, organoid cluster, sheet formation, irregular border of cluster, hyaline globule, hyaline basement membrane material, individual cell necrosis or apoptotic body, and necrotic background. Twenty cases of small cell carcinoma and fifteen cases of non-pulmonary AdCC were also reviewed for the comparison.
Results:
Statistically significant differences were identified between pulmonary AdCC and small cell carcinoma in fourteen of the fifteen cytomorphologic criteria (differences in sheet formation were not statistically significant). Cellular uniformity, distinct nucleolus, granular cytoplasm, distinct cell border, organoid cluster, hyaline globule, and hyaline basement membrane material were characteristic features of AdCC. Frequent nuclear molding, individual cell necrosis, and necrotic background were almost exclusively identified in small cell carcinoma. Although coarse chromatin and irregular cluster border were observed in both, they favored the diagnosis of small cell carcinoma. Hyaline globules were more frequently seen in non-pulmonary AdCC cases.
Adenoid cystic carcinoma (AdCC) is rare in the lower respiratory tract (less than 0.2% incidence was reported among the all primary pulmonary tumors) [1-3]. Using aspiration and exfoliative cytology for diagnosis, less than twenty cases have been reported in the English literature [4-6]. Due to its rare incidence, cytopathologic features of pulmonary AdCC have not been collectively described yet [7-11].
In salivary glands where AdCC is commonly found, cytologic characteristics of AdCC have been frequently studied and are relatively well-established. Round or ovoid nuclei and indistinct nucleoli are reported as cellular features of the AdCC. The organoid structure formed by tumor cells and hyaline globules are also helpful diagnostic features [12]. A Japanese group suggested 17 cellular and architectural features of AdCC for the cytologic diagnosis. According to the report, the AdCC could be distinguished from other salivary gland-type tumors by using the 17 items [13]. However, the subtyping of salivary gland-type tumors by fine needle aspiration (FNA) cytology is not simple, and the accuracy has been low compared to the core needle biopsy [14].
Additionally, in clinical practice, a sufficient amount of a sample is not always obtained, especially in the lower respiratory tract where the specimen acquisition by bronchoscope is usually difficult. When examining a pulmonary lesion, it is important to distinguish AdCC from other non-salivary gland-type tumors such as small cell carcinoma. Although both can share the similar cytomorphologies, the therapeutic regimens are far different [15,16]. There had been a few case reports that pulmonary AdCC was misinterpreted as small cell carcinoma [6,11].
In this study, we analyzed cytomorphologic features of 11 primary and metastatic pulmonary AdCC cases. Cytology of twenty small cell carcinomas and fifteen non-pulmonary AdCCs were also investigated for the points of differential diagnosis.
Among 93 patients who were diagnosed to have pulmonary AdCC in the Samsung Medical Center between September 1995 and June 2015, aspiration or bronchial washing cytology was performed in 36 cases. Tumor cells were identified in 11 cases and the remaining 25 cases were reported to be negative for malignant cells. The 11 cases of primary and metastatic pulmonary AdCC were all histologically confirmed as AdCC by biopsy or resection. Among the 11 AdCC cytology cases, samples for seven cases (64%) were obtained from bronchial washing specimens, and samples for four cases (36%) were acquired by endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA). Nine of the cases (82%) were obtained from trachea or bronchus samples and the remaining two cases (18%) were obtained from mediastinal lymph nodes by EBUS-TBNA. The computerized record system of Samsung Medical Center identified a total of 466 cases of small cell carcinoma which were diagnosed from cytologic specimens of mediastinal lymph nodes. Twenty cases of small cell carcinoma were randomly selected from 109 recent cases (from July 2013 to June 2015) for the cytologic comparison to pulmonary AdCC. In addition, among 426 primary AdCC cases of non-primary origin in the Samsung Medical Center between September 1995 and June 2015, both cytologic and histologic specimens were available for the review in fifteen patient cases and thus they were chosen for our study. All non-pulmonary AdCC specimens were obtained from the salivary gland or other head and neck region tumors.
Clinicopathologic information—sex, age, smoking history, site of tumor, stage, and progress was investigated by using electronic medical records. Patients were categorized into the smoker or non-smoker group according to their smoking history [17].
EBUS-TBNA and bronchial washing of the respiratory tract were performed by pulmonologists using a rigid or flexible bronchoscope. A 22-gauge needle was used in TBNA. The aspirate was smeared onto glass slides, air dried, immediately fixed with 95% alcohol and subsequently stained with hematoxylin and eosin (H&E) and a Papanicolaou solution. Bronchial washing was conducted by injecting saline solution into the bronchial tree and subsequent suctioning. The acquired washing specimen was centrifuged, fixed in a 95% alcohol and stained using H&E and a Papanicolaou solution. FNAs of the salivary gland and head and neck tumors were performed either by radiologists or pathologists using a 22- or 23-gauge needle attached to a 10-mL syringe. Ultrasonographic guidance was used in the cases performed by radiologists.
In cases that subsequent biopsies or surgical resection of the tumor were performed, histologic slides were also collected for the review.
This study was approved by the Institutional Review Board of the Samsung Medical Center (IRB No. 2015-07-154).
The cytologic slides were reviewed by three pathologists (J.H., S.K., and J.C.), including a pathologist (J.H.) who has more than 20-year experience of pulmonary pathology and cytopathology. The cases were evaluated according to fifteen cytomorphological features: (1) small cell size, (2) cellular uniformity, (3) coarse chromatin, (4) hyperchromasia, (5) distinct nucleolus, (6) frequent molding, (7) granular cytoplasm, (8) distinct individual cell border, (9) organoid cluster, (10) sheet formation, (11) irregular border of cluster, (12) hyaline globule, (13) hyaline basement membrane material, (14) individual cell necrosis or apoptotic body, and (15) necrotic background. The small cell was defined by the cell size of less than three times the diameter of the background lymphocyte. When the nuclear molding was shown in more than 50% of the entire tumor cell population, it was regarded as the frequent molding. The organoid cluster represented a cylindrical, spherical, fingerform or other distinctive architectural arrangements of tumor cells. The organoid cluster and sheet formation were indicated when more than 10% of the tumor area showed the morphologies. The above 15 features were selected by a comprehensive summary of the literature regarding AdCC and small cell carcinoma [8,12,13,18].
The histologic slides of the all pulmonary and non-pulmonary AdCC cases were also blindly reviewed.
Immunohistochemical staining of c-kit was performed to confirm the diagnosis of AdCC. Formalin-fixed, paraffin-embedded tissue samples of biopsied or resected tumors were prepared in 4-μm thickness slices and stained with a rabbit polyclonal antibody (1:50, Dako, Carpinteria, CA, USA).
Student’s t tests and Mann-Whitney tests were performed to compare the age and the duration of follow up between the pulmonary AdCC group and the small cell carcinoma group, and between the pulmonary AdCC group and the non-pulmonary AdCC group. Whether to use Student’s t test or Mann-Whitney test was decided after testing the normal distribution of data, by performing a Kolmogorov-Smirnov test and a Shapiro-Wilk test. The chi-square test, Fisher exact test, and linear-by-linear association were performed to analyze the differences in sex distribution, smoking history, stage and progression status in clinicopathologic data and the cytomorphologic items. Each test was applied in the appropriate setting. A one-tailed test was used in the case that showed unidirectional tendency. Values were considered statistically significant when the p-value was less than .05. Statistical analyses were performed by using the SPSS ver. 20 (SPSS Inc., Chicago, IL, USA).
The clinical and pathological characteristics of the 11 pulmonary AdCC cases are summarized in Table 1. Patients included in the pulmonary AdCC group were six males and five females and the median age was 57 years old. Among the nine patients whose smoking histories were available, five patients were smokers (median, 12.5 pack-years; range, 10 to 30 pack-years). Most of the pulmonary AdCC cases were observed as obstructive masses or narrowing of trachea/bronchus in bronchoscopy. Except for the one case that lacked staging information, stage I, II, III, and IV constituted 20%, 10%, 60%, and 10% of the cases, respectively. In three cases (27%), the tumors had recurred on their original sites. Metastasis was identified in two patients during follow-up (18%).
Only one case (9%) was confirmatively diagnosed as AdCC in the cytologic specimen (case 3). Eight cases (73%) were diagnosed as malignancy or suspicious for malignancy but specific types were not mentioned. The remaining two cases (18%) were diagnosed as the presence of atypical cells.
All cases were confirmed as AdCC by subsequent biopsies and surgical resections. Nine cases (82%) showed a cribriform pattern of growth, whereas the remaining two cases (18%) showed a solid pattern.
Clinical features of the selected small cell carcinoma cases and the non-pulmonary AdCC cases are summarized in Table 2. Among the small cell carcinoma cases, 15 cases (75%) were obtained from N1 mediastinal lymph nodes. Three cases (15%) were acquired from N2 lymph nodes. Eleven patients (55%) were in the limited stage of disease whereas the remaining nine patients (45%) were in the extensive stage. Tumor recurrence was identified in two patients (10%). The patients’ age at diagnosis was older and the follow-up period was shorter in the patients with small cell carcinoma compared to the patients with pulmonary AdCC. All except one of the small cell carcinoma patients had a smoking history (median, 45 pack-years; range, 30 to 112.5 pack-years).
Non-pulmonary AdCC cases were all obtained by FNA. Twelve cases (80%) were taken from either parotid or submandibular glands. Two cases (13%) were the tumors of external auditory canal. One case (7%) was obtained by FNA of the floor of the mouth. Sex ratio, median age, smoking history (median, 33.75 pack-years; range, 22.5 to 45 pack-years in smoker group), stage, presence of recurrence or metastasis and follow-up period were not statistically different from those of the pulmonary AdCC group.
The fifteen cytomorphologic criteria described above were evaluated in the pulmonary and the non-pulmonary AdCC and the small cell carcinoma cases (Table 3). Except for sheet formation, all other cytomorphologic criteria showed statistically significant differences between pulmonary AdCC and small cell carcinoma. Although 45% of the pulmonary AdCC cases manifested small cell size, the tumor cells were cytologically uniform and they frequently had distinct nucleoli which were not noted in small cell carcinoma. While six cases (55%) of pulmonary AdCC revealed a coarse chromatin pattern, it was identified in all but one case of small cell carcinoma. Nucleoli were distinct in six cases (55%) of pulmonary AdCC, but all of the small cell carcinoma cases showed indistinct nucleoli. Nuclear molding was occasionally identified in seven out of 10 available pulmonary AdCC cases (70%). However, none exceeded 50% of the entire tumor cell population. In contrast, the extensive nuclear molding was noted in 95% of the small cell carcinoma cases. Many pulmonary AdCC cases had tumor cells with scant cytoplasm and/or naked nuclei similar to small cell carcinoma. However, rigorous microscopic examination revealed granular cytoplasm in the majority of the cases (73%). The cellular border was distinct in the three pulmonary cases (27%), while none of the small cell carcinoma cases showed distinguishable cellular borders. Regarding the architectural patterns, organoid cluster formation was observed in all of the pulmonary AdCC cases, but two small cell carcinoma cases also revealed vaguely organoid clusters. Whereas the border of cell clusters was irregular in all of the small cell carcinoma cases, regular borders were identified in 56% of the pulmonary AdCC cases. Hyaline globules and hyaline basement membrane materials were exclusively identified in the pulmonary AdCC cases; however, the frequency is lower than expected (36% and 27%, respectively). The single cell necrosis or apoptotic body was not observed in any pulmonary AdCC case. Although the two pulmonary AdCC cases (18%) revealed necrotic background, it was identified in 80% of small cell carcinoma cases (Figs. 1, 2). In summary, cellular uniformity, distinct nucleolus, granular cytoplasm, distinct cell border, organoid cluster, hyaline globule and hyaline basement membrane were revealed as cytologic features of pulmonary AdCC compared to small cell carcinoma. Coarse chromatin, frequent nuclear molding, irregular border of cluster, individual cell necrosis or apoptotic body, and necrotic background could be considered as characteristic cytologic findings of small cell carcinoma.
When comparing pulmonary AdCC to non-pulmonary AdCC cases according to the fifteen cytomorphologic features, small cell size and sheet formation were less frequently observed in the pulmonary AdCC cases. Whereas 80% of the non-pulmonary AdCC cases had hyaline globules, they were only seen in 36% of the pulmonary AdCC cases.
The review of biopsy or surgical resection slides of the 11 pulmonary AdCC cases revealed that nine cases had a cribriform growth pattern while two cases showed a solid pattern of growth. Tumors with a solid pattern of growth were confirmed by immunohistochemical staining with c-kit (data not shown). In the non-pulmonary AdCC cases, 11 cases (79%) showed cribriform predominant histology, while the tubular and solid growth patterns were noted in the rest cases (14% and 7%, respectively).
Pulmonary AdCC is often difficult to diagnose based on cytologic analysis. Besides the fact that they frequently show similar cytomorphology, many primary tumors of the lung share common clinical features. Therefore, differential diagnosis often includes a variety of malignant tumors such as small cell carcinoma, carcinoid tumor and poorly differentiated non-small cell carcinoma. Salivary gland type tumors other than AdCC-pleomorphic adenoma, myoepithelial adenoma/carcinoma, basal cell adenoma/carcinoma and epithelial-myoepithelial carcinoma should also be considered [13].
Small cell carcinoma is an especially important differential diagnosis to consider because its treatment and prognosis is far different from AdCC. Small cell carcinoma has been thought to be distinguished from AdCC in cytology by nuclear molding and necrosis. However, research has demonstrated the occasional presence of such features in AdCC as well [6,11,19]. From these reports, pulmonary AdCC often demonstrated small cell size, coarse chromatin [13] and occasional nuclear molding [11]. Chuah et al. [10] also demonstrated the diagnostic difficulty of AdCC in bronchial washing by emphasizing the importance of the clean background of AdCC compared to the necrotic background of small cell carcinoma. Kim et al. [6], who reported a misinterpreted case of AdCC, suggested that lack of apoptotic bodies, nuclear debris, frequent mitoses, and the Azzopardi effect could be distinctive points between AdCC and small cell carcinoma.
In this study, pulmonary AdCC and small cell carcinoma were well-distinguished from each other when applying the 15 cytomorphological features that we proposed. With the exception of sheet formation, the remaining fourteen features showed statistically significant differences. Cellular uniformity (90%), granular cytoplasm (73%), and organoid cluster formation (100%) were identified as distinguishable features of pulmonary AdCC compared to small cell carcinoma. However, some pulmonary AdCC cases contained cytomorphologic features of small cell carcinoma-coarse chromatin (55%), indistinct nucleolus (55%), occasional molding (70%), and necrotic background (18%). Therefore, one should not depend on a single criterion to conclude the diagnosis in cytology of the pulmonary tumor. Instead, a comprehensive approach using multiple cytomorphological features is recommended. Among the clinical items we investigated, smoking history was the most distinguishable between the pulmonary AdCC group and the small cell carcinoma group. Patients with small cell carcinoma had significant smoking history. Also, the small cell carcinoma cases showed extensive involvement of mediastinal lymph nodes compared to the pulmonary AdCC cases. These clinical presentations could also be helpful for confirmative diagnosis.
Confronting the individual cases of pulmonary AdCC, there were several points that could be confused with small cell carcinoma. Tumor cells of case 1 in Table 1 were small and they had indistinct nucleoli and occasional nuclear molding (in about 20% of total tumor volume) (Fig. 2B). Although the chromatin pattern was fine-stippled rather than coarse, and granular cytoplasm was identified in some tumor cells, it was difficult to exclude small cell carcinoma straightforwardly (Fig. 2A). Furthermore, only a small portion of the cytology specimens revealed organoid clusters. The biopsy specimen of case 1 also showed small cell carcinoma-like morphology which was observed in the cytologic slides (Fig. 2C). Recognition of cellular uniformity and focal hyaline basement membrane material was essential in the case. Case 3 in Table 1 consisted of small cells with fine-stippled to coarse chromatin and paucity of granular cytoplasm (Fig. 2E). However, the tumor cells were relatively uniform and lacked nuclear molding compared to small cell carcinoma. Careful microscopic examination confirmed the diagnosis by identifying organoid clusters with hyaline basement membrane material. A bronchial washing case (case 6 in Table 1) showed a few tumor cells scattered in the necrotic background (Fig. 2H). Although the cell size was slightly larger than small cells and the chromatin pattern was fine rather than coarse, small cell carcinoma was considered in the differential diagnosis due to the necrotic background. However, background necrosis was far more evident in the small cell carcinoma cases, and the individual cell necrosis and the apoptotic bodies were frequently identified (Fig. 2G). The review of histologic slides obtained later by surgical resection revealed ulceration in the surface of the mass with numerous inflammatory cells (Fig. 2I).
We were suspicious of the low frequency of hyaline globules and hyaline basement membrane materials in the pulmonary AdCC cases, as we thought they were stereotypical features of the AdCC. We therefore randomly selected non-pulmonary AdCC cases and concomitantly evaluated them according to the 15 cytomorphologic features we identified. Interestingly, small cell size was significantly more common in non-pulmonary AdCC. Compared to the non-pulmonary AdCC cases, pulmonary AdCC was less likely to form diffuse sheets or to have hyaline globules. The lack of hyaline globules may make it difficult to diagnose AdCC, especially when the specimen cellularity is low enough so that architectural information specific for AdCC is not available.
A previous study identified a solid variant of pulmonary AdCC that exhibited tumor cell clusters, which neither formed cylinders/spheres nor were sharply demarcated in cytology [8]. Two out of 11 cases included in this study showed the solid pattern of growth in biopsy or resection specimens (cases 1 and 2 in Table 1). However, even though the proportion of organoid clusters was relatively small, both cases had the features that favored the diagnosis of AdCC; cellular uniformity, distinct nucleolus (in case 2 only), granular cytoplasm and hyaline basement membrane material (in case 1 only). Furthermore, lack of frequent molding, single cell necrosis and necrotic background precluded the diagnosis of small cell carcinoma.
We recommend using the fifteen diagnostic features not only in the diagnosis of pulmonary AdCC, but also in the cytologic diagnosis of the other AdCC cases. The diagnosis of metastatic AdCC in lymph nodes or other distant organs may not be straightforward because typical architectures such as cribriform or tubular pattern may not be identified in metastatic lesions [18-23]. According to the Yu and Caraway [20] who reviewed the FNA findings of metastatic AdCC, five cases (62.5%) had a solid arrangement of tumor cells on FNA slides which made it difficult to diagnose.
In summary, we have identified fifteen cytomorphologic features that could be used to successfully distinguish pulmonary AdCC from small cell carcinoma. In this study, we noticed that pulmonary AdCC might be misinterpreted as small cell carcinoma when only a single or a few cytologic features were considered. Therefore, a comprehensive analysis of morphologic features is required in the cytologic diagnosis of pulmonary AdCC.
REFERENCES
1. Inoue H, Iwashita A, Kanegae H, Higuchi K, Fujinaga Y, Matsumoto I. Peripheral pulmonary adenoid cystic carcinoma with substantial submucosal extension to the proximal bronchus. Thorax. 1991; 46:147–8.

2. Moran CA, Suster S, Koss MN. Primary adenoid cystic carcinoma of the lung: a clinicopathologic and immunohistochemical study of 16 cases. Cancer. 1994; 73:1390–7.

3. Maziak DE, Todd TR, Keshavjee SH, Winton TL, Van Nostrand P, Pearson FG. Adenoid cystic carcinoma of the airway: thirty-two-year experience. J Thorac Cardiovasc Surg. 1996; 112:1522–31.

4. Cho YM, Park SY, Lee IC. Cytopathologic features of adenoid cystic of trachea carcinoma: report of 2 cases. Korean J Cytopathol. 1995; 6:214–8.
5. Lee JS, Kim JS, Yang BS, Lee MC, Park CS, Juhng SW. Cytopathologic features of primary bronchial adenoid cystic carcinoma: a case report. Korean J Cytopathol. 1995; 6:67–70.
6. Kim HJ, Choi S, Kwon J, Kim JY, Park K. Bronchial brushing cytologic finding of primary pulmonary adenoid cystic carcinoma misinterpretated as small cell carcinoma: a case report with literature review. Korean J Pathol. 2011; 45:441–4.
7. Qiu S, Nampoothiri MM, Zaharopoulos P, Logroño R. Primary pulmonary adenoid cystic carcinoma: report of a case diagnosed by fine-needle aspiration cytology. Diagn Cytopathol. 2004; 30:51–6.

8. Ozkara SK, Turan G. Fine needle aspiration cytopathology of primary solid adenoid cystic carcinoma of the lung: a case report. Acta Cytol. 2009; 53:707–10.
9. Segletes LA, Steffee CH, Geisinger KR. Cytology of primary pulmonary mucoepidermoid and adenoid cystic carcinoma: a report of four cases. Acta Cytol. 1999; 43:1091–7.
10. Chuah KL, Lim KH, Koh MS, Tan HW, Yap WM. Diagnosis of adenoid cystic carcinoma of the lung by bronchial brushing: a case report. Acta Cytol. 2007; 51:563–6.
11. Daneshbod Y, Modjtahedi E, Atefi S, Bedayat GR, Daneshbod K. Exfoliative cytologic findings of primary pulmonary adenoid cystic carcinom: a report of 2 cases with a review of the cytologic features. Acta Cytol. 2007; 51:558–62.
12. Klijanienko J, Vielh P. Fine-needle sampling of salivary gland lesions. III. Cytologic and histologic correlation of 75 cases of adenoid cystic carcinoma: review and experience at the Institut Curie with emphasis on cytologic pitfalls. Diagn Cytopathol. 1997; 17:36–41.

13. Hara H, Oyama T, Suda K. New criterial for cytologic diagnosis of adenoid cystic carcinoma. Acta Cytol. 2005; 49:43–50.
14. Song IH, Song JS, Sung CO, et al. Accuracy of core needle biopsy versus fine needle aspiration cytology for diagnosing salivary gland tumors. J Pathol Transl Med. 2015; 49:136–43.

15. Szyfelbein WM, Ross JS. Carcinoids, atypical carcinoids, and small-cell carcinomas of the lung: differential diagnosis of fine-needle aspiration biopsy specimens. Diagn Cytopathol. 1988; 4:1–8.

16. Delgado PI, Jorda M, Ganjei-Azar P. Small cell carcinoma versus other lung malignancies: diagnosis by fine-needle aspiration cytology. Cancer. 2000; 90:279–85.
17. Lee JS, Hirsh V, Park K, et al. Vandetanib Versus placebo in patients with advanced non-small-cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: a randomized, double-blind phase III trial (ZEPHYR). J Clin Oncol. 2012; 30:1114–21.

18. Anderson RJ, Johnston WW, Szpak CA. Fine needle aspiration of adenoid cystic carcinoma metastatic to the lung: cytologic features and differential diagnosis. Acta Cytol. 1985; 29:527–32.
19. David D, Masineni SN, Giorgadze T. Fine-needle aspiration diagnosis of high grade adenoid cystic carcinoma metastatic to the pancreas. Diagn Cytopathol. 2015; 43:117–20.

20. Yu GH, Caraway NP. Poorly-differentiated adenoid cystic carcinoma: cytologic appearance in fine-needle aspirates of distant metastases. Diagn Cytopathol. 1996; 15:296–300.

21. Mardi K, Kaushal V, Uppal H. Cytodiagnosis of intracranial metastatic adenoid cystic carcinoma: spread from a primary tumor in the lacrimal gland. J Cytol. 2011; 28:200–2.

22. Smith RC, Amy RW. Adenoid cystic carcinoma metastatic to the lung: report of a case diagnosed by fine needle aspiration biopsy cytology. Acta Cytol. 1985; 29:533–4.
23. Park SY, Lee KG. Metastatic adenoid cystic carcinoma of the lung diagnosed by fine needle aspiration biopsy. Korean J Cytopathol. 1990; 1:175–8.
Fig. 1.
Cytomorphology of pulmonary adenoid cystic carcinoma. (A) Small cell size, cellular uniformity and hyperchromasia (case 1). (B) Infrequently identified nuclear molding (case 7). (C) Granular cytoplasm and well-defined cell borders (case 7). (D) Distinct nucleoli and sheet formation (case 2). (E) Organoid tumor clusters with smooth border (case 9). (F) Hyaline globules (case 3). (G) Hyaline basement membrane materials (case 3). (H) Necrotic background (case 6).
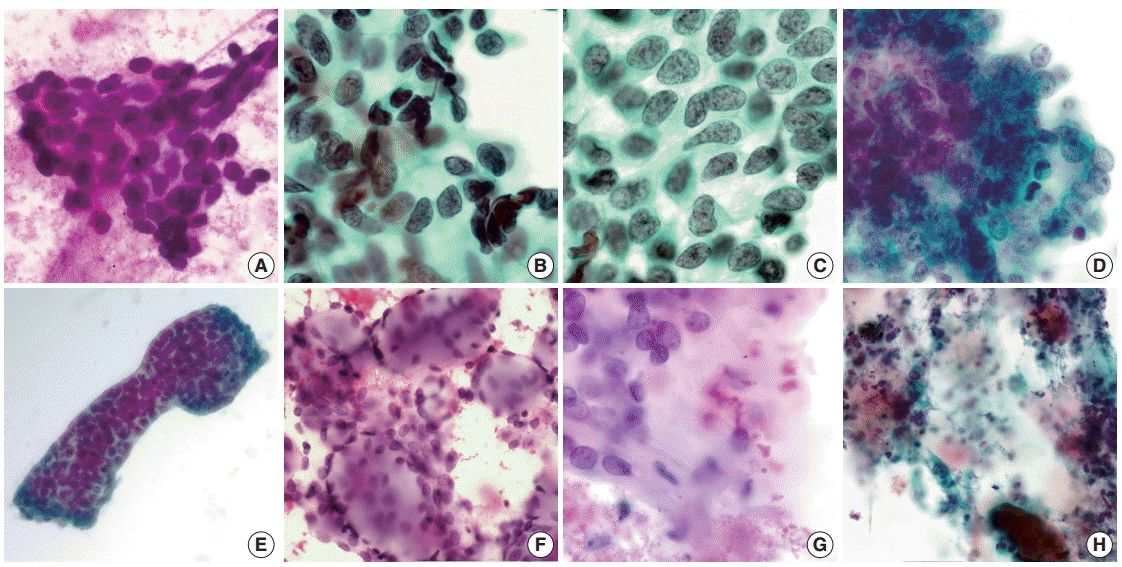
Fig. 2.
Cytologic comparison between pulmonary adenoid cystic carcinoma (AdCC) and small cell carcinoma with histologic confirmation. (A) Lack of cellular uniformity in small cell carcinoma. (B) Uniform tumor cells of pulmonary AdCC with occasional nuclear molding (case 1). (C)Biopsy specimen of pulmonary AdCC case 1, which mimicked small cell carcinoma morphology. (D) Coarse chromatin pattern with frequent nuclear molding in small cell carcinoma. (E) Size variation of the tumor cells with fine-stippled to coarse chromatin in pulmonary AdCC (case3). (F) Biopsy specimen showing typical histology of AdCC (case 3). (G) Extensively necrotic background with frequent single cell necrosis and apoptotic bodies in the small cell carcinoma aspirate. (H) Necrotic background without individual tumor cell necrosis or apoptotic body in pulmonary AdCC (case 6). (I) Surface ulceration identified in the resection specimen of pulmonary AdCC (case 6).
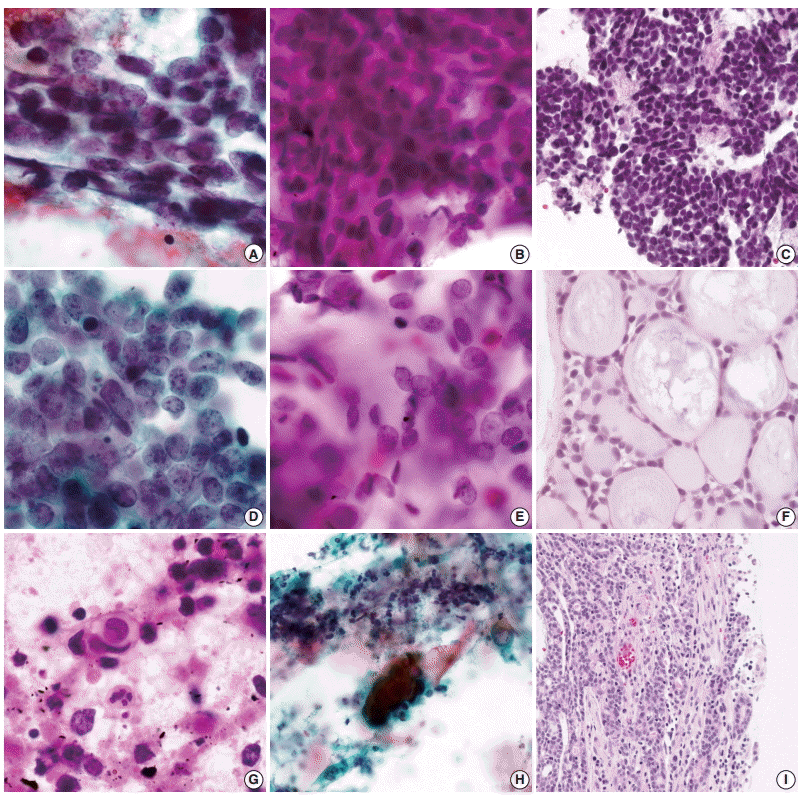
Table 1.
Clinicopathologic characteristics of 11 pulmonary adenoid cystic carcinoma cases
Table 2.
Clinical characteristics of pulmonary adenoid cystic carcinoma, metastatic small cell carcinoma, and non-pulmonary adenoid cystic carcinoma cases
| Characteristic | Pulmonary AdCC (n = 11) | SC (n = 20) | Non-pulmonary AdCC (n = 15) | p-value |
|---|---|---|---|---|
| Sex | ||||
| Male | 6 (55) | 18 (90) | 6 (40) | .067a,b |
| Female | 5 (45) | 2 (10) | 9 (60) | .462c,d |
| Median age (range, yr) | 57 (42–75) | 68 (57–77) | 53 (32–76) | < .001a,e |
| .168c,e | ||||
| Smoking history | ||||
| Smokerf | 5 (56)g | 19 (95) | 2 (14)g | .022a,b |
| Never smokerh | 4 (44)g | 1 (5) | 12 (86)g | .066c,b |
| Site | Trachea: 4 (36) | N1 LN: 15 (75) | Salivary gland: 12 (80) | - |
| Bronchus: 5 (46) | N2 LN: 3 (15) | EAC: 2 (13) | ||
| Mediastinal LN: 2 (18) | Unspecified: 2 (10) | Mouth floor: 1 (7) | ||
| Stage | I: 2 (20)i | Limited: 11 (55) | I: 3 (20) | .667c,j |
| II: 1 (10)i | Extensive: 9 (45) | II: 4 (27) | ||
| III: 6 (60)i | III: 1 (7) | |||
| IV: 1 (10)i | IV: 7 (46) | |||
| Recurrence or metastasis | ||||
| Present | 5 (45) | 2 (10) | 5 (33) | .067a,d |
| Absent | 6 (55) | 18 (90) | 10 (67) | .689c,d |
| Median follow up (range, mo) | 48 (3–177) | 7.5 (1–19) | 41 (4–70) | .003a,k |
| .264c,e |
Values are presented as number (%) unless otherwise indicated.
AdCC, adenoid cystic carcinoma; SC, small cell carcinoma; LN, lymph node; EAC, external auditory canal.
f Smoker group includes ex-smoker (stopped smoking for more than 365 days), habitual smoker (more than 1 tobacco product/day) and occasional smoker (less than 1 tobacco product/day);
g Smoking history was not able to obtain in two pulmonary AdCC cases and one non-pulmonary AdCC case;
Table 3.
Cytomorphologic features of pulmonary AdCC, SC, and non-pulmonary AdCC
| Feature | Pulmonary AdCC (n=11) | SC (n=20) | Non-pulmonary AdCC (n=15) | p-value (pulmonary AdCC/SC) | p-value (pulmonary AdCC/non-pulmonary AdCC) |
|---|---|---|---|---|---|
| Histologic pattern | |||||
| AdCC, tubular pattern | 0/11 | - | 2/14 | - | .167a |
| AdCC, cribriform pattern | 9/11 | - | 11/14 | ||
| AdCC, solid pattern | 2/11 | - | 1/14 | ||
| Cytologic findings | |||||
| Specimen cellularity | .031a | .138a | |||
| 3 | 3/11 | 13/20 | 9/15 | ||
| 2 | 4/11 | 5/20 | 4/15 | ||
| 1 | 4/11 | 2/20 | 2/15 | ||
| Small cell | 5/11 | 20/20 | 13/15 | .001b | .038b |
| Cellular uniformity | 9/10 | 2/20 | 12/15 | < .001b | .626b |
| Coarse chromatin | 6/11 | 19/20 | 7/15 | .013b | .691c |
| Hyperchromasia | 5/11 | 20/20 | 12/15 | .001b | .103b |
| Distinct nucleolus | 6/11 | 0/20 | 10/15 | .001b | .689b |
| Frequent molding | 0/11 | 19/20 | 1/15 | < .001b | .577b |
| Granular cytoplasm | 8/11 | 0/20 | 12/15 | < .001b | .509b |
| Distinct cell border | 3/11 | 0/20 | 4/15 | .037b | .655b |
| Organoid cluster | 8/8 | 2/20 | 12/15 | < .001b | .526b |
| Sheet formation | 4/9 | 13/20 | 14/15 | .422b | .015b |
| Irregular cluster border | 4/9 | 20/20 | 7/15 | .001b | .625b |
| Hyaline globule | 4/11 | 0/20 | 12/15 | .010b | .043b |
| Hyaline BM material | 3/11 | 0/20 | 5/15 | .037b | .543b |
| Cell necrosis/apoptotic body | 0/11 | 18/20 | 2/15 | < .001b | .492b |
| Necrotic background | 2/11 | 16/20 | 2/15 | .002b | .574b |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download